 Over the last two decades, the pharmaceutical industry has faced increasing challenges: declining R&D productivity, rising costs, competition from generics, greater regulatory complexity, and changing expectations of patient-centered healthcare. In response to these pressures, many companies are transforming the way in which medicines are discovered, developed, and brought to market.
Over the last two decades, the pharmaceutical industry has faced increasing challenges: declining R&D productivity, rising costs, competition from generics, greater regulatory complexity, and changing expectations of patient-centered healthcare. In response to these pressures, many companies are transforming the way in which medicines are discovered, developed, and brought to market. Two key trends are emerging:
- The proliferation of digital biotechs
- The externalization of drug development
Contract Research and Drug Development
Digital biotechs represent a new contract research model for developing medicines, where pharma and biotech companies collaborate under more data-driven, agile development processes and leverage external collaborators to drive the development of novel therapies.This new pharmaceutical R&D paradigm is highly collaborative and externalized. Pharma focuses their resources on late-stage development and commercialization, outsourcing the bulk of early-stage discovery and development work to academia and CROs.
This new model promises to simultaneously expedite the development of new medicines and lower overall costs. In our view, however, the critical sponsor-CRO relationship has not evolved with this reality.
At Taconic Biosciences and TetraScience, we are seeing a new type of "CRO" emerge in response. We call these entities "Collaborative Research Organizations".
What are Collaborative Research Organizations?
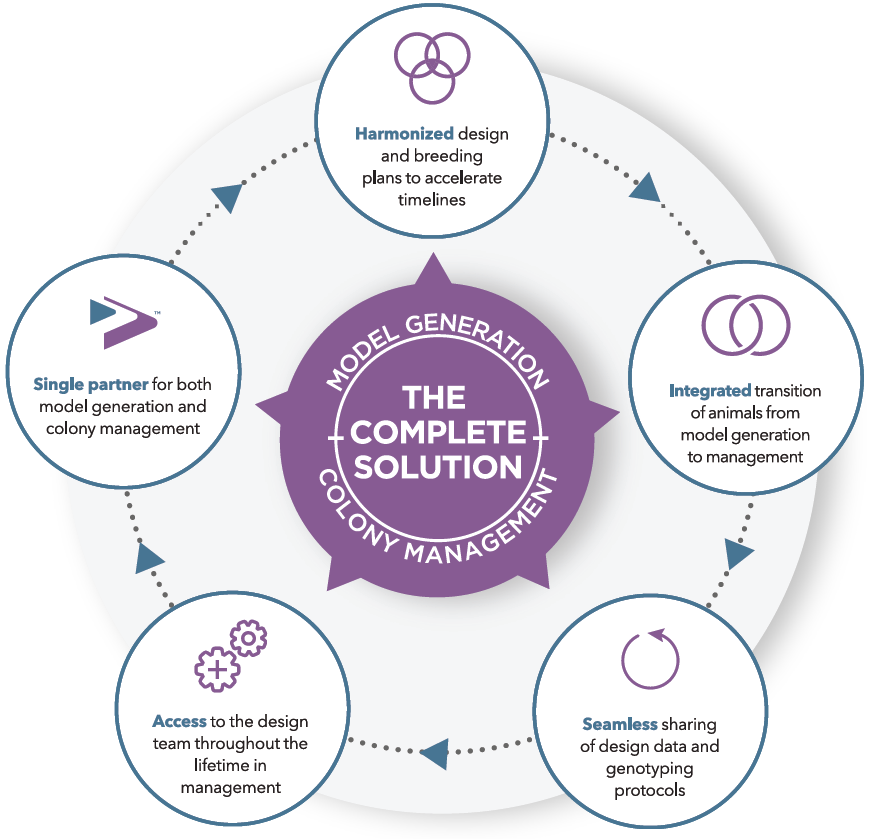
This new form of CRO is reshaping outsourcing models of contract research (Figure 1), moving away from the traditional "fee-for-service" arrangement (where price dominates) and towards strategic partnerships where risks and profits are shared. Ultimately, this produces more integrated relationships, where sponsors and CROs co-create value through increased alignment and efficiency.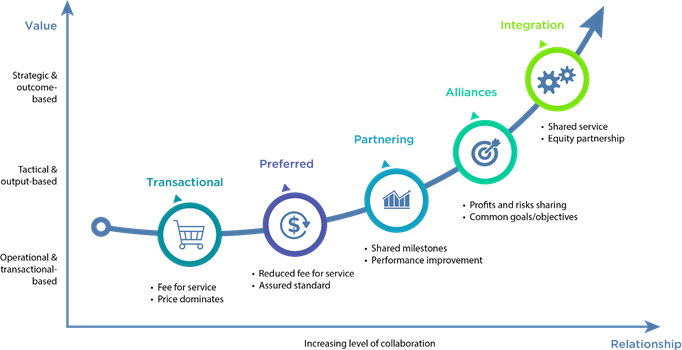
Figure 1: Evolution of outsourcing models in drug development
Defining Collaborative Research Organizations
A Collaborative Research Organization has the following characteristics:WIIFWE Mindset
Thriving in today's turbulent, rapidly evolving R&D landscape requires a vested, collaborative mindset based on What's-In-It-For-We (WIIFWE). This is vastly different from the prevailing zero-sum philosophy, where one party captures all the benefits at the expense of others. A WIIFWE mindset focuses on creating mutually symbiotic relationships where both parties are equally committed to each other's success.Collaborative research organizations adopt this expansive view and focus on working together with sponsoring organizations to get medicines to market faster, more cost-effectively, and with fewer risks. According to research conducted by the University of Tennessee, a win-win mindset is the foundation for the most successful and enduring business relationships, driving innovation and game-changing results.
Data-centric
Collaborative research organizations develop big data analytical capabilities to handle the volume, variety, and velocity of scientific data on behalf of clients, allowing information to be translated into strategic insights. Through the use of big data, these organizations create value in drug discovery and development previously unimaginable. Here are some examples:- Data-rich communication and collaboration can help clients have deeper view in to the science and more robust data sets for internal decision making.
- Data-driven predictive modeling can help clients to identify new candidate molecules with promising safety and efficacy profile to increase the chance of success.
- Data-centric operational models that ensure customers have greater visibility and faster turnaround times for mission-critical studies.
Becoming a Collaborative Research Organization
Build mutual trust and respect
Successful sponsor-CRO collaboration requires open communication to create a shared vision based on mutual trust and respect. At the beginning of the partnership, it is important to clarify objectives, expectations, responsibilities, commitments, and investments for each party to ensure the sponsor and the CRO are fully aligned.Adopt modern data tools
A collaborative partnership also needs a high degree of cross-organizational transparency and information sharing. By using modern data tools, CROs can facilitate the rapid exchange of documents, workflows, and common performance metrics with sponsors to increase operational efficiency, improve resource deployment, and reduce cycle times.Integrate processes and systems
Another key element of the collaborative relationship is full integration, where the CRO functions as a true extension of the sponsor. CROs bring deep therapeutic and clinical expertise to create efficient study design and operational models. When processes and systems are well-integrated, sponsors and CROs work together seamlessly and significant cost and time-saving benefits can be realized.Have a governance structure
A collaborative relationship is based on the principle of shared risks and rewards. A governance structure with roles and responsibilities for execution and oversights clearly defined fosters upward and downward communication and provides visibility of key metrics across divisions. This is fundamental to strong performance, issue escalation, and risk management.










.jpg)

.jpg)
.jpg)
.jpg)
.jpg)



.jpg)


.jpg)
.jpg)


.jpg)


.jpg)

.jpg)




