Application Areas:
huNOG-EXL SA (Standard Access)

| Model No. | Nomenclature | Genotype |
|---|---|---|
| HSCCB-13395-F | NOD.Cg-Prkdcscid Il2rgtm1Sug Tg(SV40/HTLV-IL3,CSF2)10-7Jic/JicTac | sp/sp;ko/ko;tg/wt |
- Description
- Data
- Price & Licensing
- Health Report
- Overview
- Genetics
- Guides & Publications
- Applications & Therapeutic Areas
- Transit, Housing & Welfare
- Diet
Overview
A Humanized Immune System (HIS) Mouse Supporting Both Myeloid & Lymphoid Cells
Nomenclature: NOD.Cg-Prkdcscid Il2rgtm1Sug Tg(SV40/HTLV-IL3,CSF2)10-7Jic/JicTac
For researchers who want access to live inventory for immediate delivery, the huNOG-EXL SA (Standard Access) is the ideal model, particularly for experiments with shorter timelines or studies that must start as soon as possible.
Taconic's portfolio of HIS mice includes both the huNOG-EXL SA (Standard Access) as well as the huNOG-EXL EA (Early Access). Both SA and EA models are available with simple terms of use and are individually applicable for different research goals.
- Humanized immune system mouse generated through engraftment of human CD34+ hematopoietic stem cells (HSCs) in NOG-EXL mice, available as an inventoried, study-ready product.
- All mice shipped meet a specified QC standard of ≥25% hCD45 in peripheral blood at 10 weeks post-engraftment.
- NOG-EXL host strain is a super immunodeficient NOG mouse expressing human GM-CSF and human IL-3 cytokines to support myeloid lineage engraftment.
- Higher overall engraftment levels of human hematopoietic stem cells (HSC) compared to core NOG mouse. In most cases, huNOG-EXL mice average >40% human cells in blood.
- Higher levels of myeloid cell differentiation following human HSC engraftment compared to core NOG mouse.
- Applications in research involving oncology and immuno-oncology, autoimmune disease, allergy, infectious disease, immunology, regenerative medicine, safety assessment, and humanization.
- Humanized immune system mice require special housing and husbandry. We recommend reviewing the the comprehensive document Licensing, Care & Resources for Taconic's Humanized Immune System Models and the Video: Care of HIS mice for experimental success prior to ordering. We strongly recommend that customers schedule a complimentary consultation with a Field Application Scientist prior to ordering so as to maximize experimental success.
Which huNOG-EXL is right for you?
| huNOG-EXL SA | huNOG-EXL EA | |
|---|---|---|
| Price | ++ | + |
| Timeline | Inventoried at 10+ WPE and ready to go on study after a short acclimation | 5-6 week order lead time; shipped 1-2 WPE |
| Available for experimental manipulation prior to 10 WPE | No | Yes |
| Chimerism QC data available | Yes | No |
| Who assumes the risk that individual animals fail to engraft? | Taconic | Customer |
| % mice guaranteed to meet QC standard | 100% | N/A |
| Maximum n-value per donor? | 35-40 mice | 35-40 mice |
| Minimum purchase | No minimum | 20 mice |
| Cancellation policy | Cancellation deadline 4 weeks prior to ship date | May not be canceled after order is booked |
Availability
- Taconic maintains an inventory of female huNOG-EXL mice engrafted as juveniles and FACS tested at 10 weeks post-engraftment.
- Custom options such as HLA selection for huNOG-EXL Standard Access are available with additional fee. Inquire regarding your specific needs.
- Orders with a specification regarding number of mice per donor are subject to availability and are not guaranteed, as orders may need to be adjusted at time of packing.
Lifespan: All myeloid-supportive HIS mice have limited lifespans due to a range of outcomes including anemia, thrombocytopenia, macrophage activation syndrome and hemophagocytic lymphohistiocytosis. The huNOG-EXL has the longest demonstrated lifespan compared to competing models, but this varies by donor and can be impacted by environmental and experimental factors. While Taconic has observed that huNOG-EXL mice can often survive for 25+ weeks post-engraftment (WPE), researchers should plan for studies to be complete by 23-26 WPE.
Origin
Genetics
Guides & Publications
Initial Publication:
- Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, Tsuji K, Heike T, Nakahata T. (2002) NOD/SCID/γ
 mouse: an excellent recipient mouse model for engraftment of human cells. Blood 100(9):3175-3182.
mouse: an excellent recipient mouse model for engraftment of human cells. Blood 100(9):3175-3182. - Fukuchi Y, Miyakawa Y, Kobayashi K, Kuramochi T, Shimamura K, Tamaoki N, Nomura T, Ueyama Y, Ito M. (1998) Cytokine dependent growth of human TF-1 leukemic cell line in human GM-CSF and IL-3 producing transgenic SCID mice. Leuk Res 22(9):837-43.
- Ito R, Takahashi T, Katano I, Kawai K, Kamisako T, Ogura T, Ida-Tanaka M, Suemizu H, Nunomura S, Ra C, Mori A, Aiso S, Ito M. (2013) Establishment of a human allergy model using human IL-3/GM-CSF-transgenic NOG mice. J Immunol.191(6):2890-9.
Applications & Therapeutic Areas
- Immunology
- Infectious Disease
- Oncology & Immuno-Oncology
- Safety Assessment
- Cell and Tissue Humanized
- Inflammation
Transit, Housing & Welfare
Need more info? Click the live chat button or Contact Us
Taconic packs humanized immune system mice as cagemates - one engraftment lot per Taconic Transit Cage (TTC). Taconic does not combine different lots into a single TTC.
Diet
Data
huNOG-EXL Mice Have Human Myeloid Cells

Figure 1: Flow cytometry of peripheral blood from a representative huNOG-EXL mouse at 10 weeks-post engraftment demonstrates the presence of human myeloid cells (hCD45+hCD33+).
Kinetics of Human Immune Cell Reconstitution in huNOG-EXL
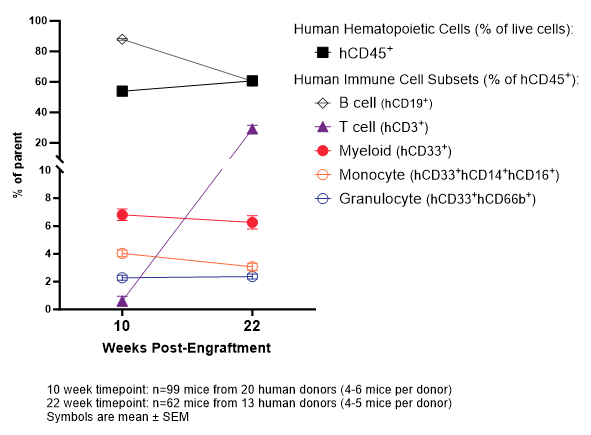
Figure 2: Kinetics of human immune cell reconstitution in huNOG-EXL (peripheral blood). huNOG-EXL mice support high levels of human immune cells in peripheral blood (chimerism) and develop both myeloid and lymphoid cells. huNOG-EXL EA mice are shipped shortly after engraftment, prior to reconstitution of significant numbers of human immune cells in peripheral blood. huNOG-EXL SA mice are shipped after a QC step to confirm that each mouse has ≥25% human cells in peripheral blood at 10 weeks post-engraftment.
huNOG-EXL mice have extended lifespan compared to competing models
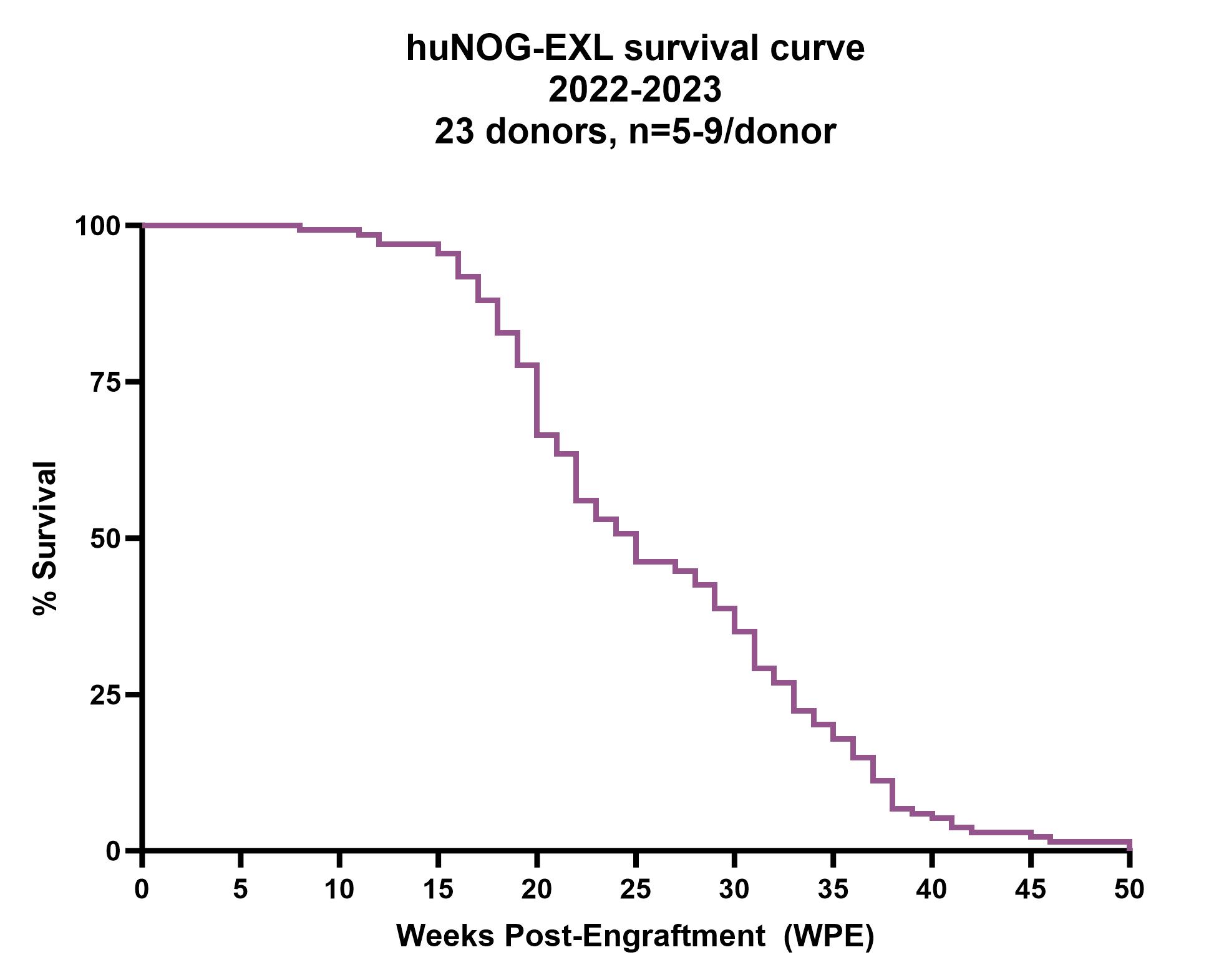
Figure 3: Survival curve for huNOG-EXL mice across 23 donors (n=5-9 per donor), generated in the Taconic Biosciences humanization core during 2022-2023. All myeloid-supportive HIS mice have limited lifespans due to a range of outcomes including anemia, thrombocytopenia and myeloid cell hyperactivation syndrome. huNOG-EXL has the longest demonstrated lifespan compared to competing models; this varies by donor and can be impacted by environmental and experimental factors. Internal Taconic data.
How Taconic Addresses HIS Mouse Variability
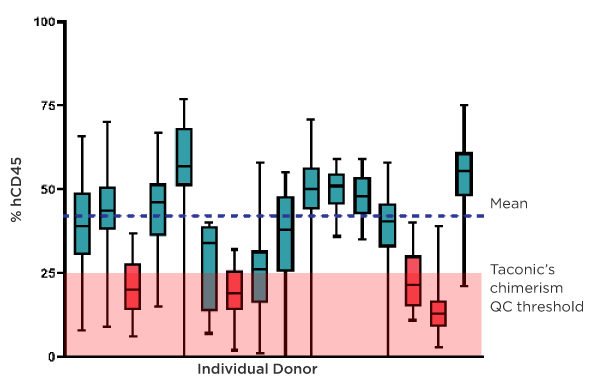
Figure 4: Donor characteristics impact human immune system reconstitution in huNOG-EXL mice, including chimerism levels and frequencies of specific human immune cell types. For huNOG-EXL SA, lots for which the mean falls below Taconic's 25% chimerism threshold are disqualified from sale (lots shown in red). For lots with a mean above the threshold (shown in blue), any individual animals in the lot which score below 25% are disqualified from sale. For huNOG-EXL EA, Taconic selects donors that have previously been validated to reduce the risk of lot failure. Taconic's Field Application Scientists can help you design a successful study plan which appropriately accounts for both inter- and intra-donor variation in HIS mice.
huNOG-EXL EA Mice are Engrafted Using Donor Cells with Proven Engraftment Success
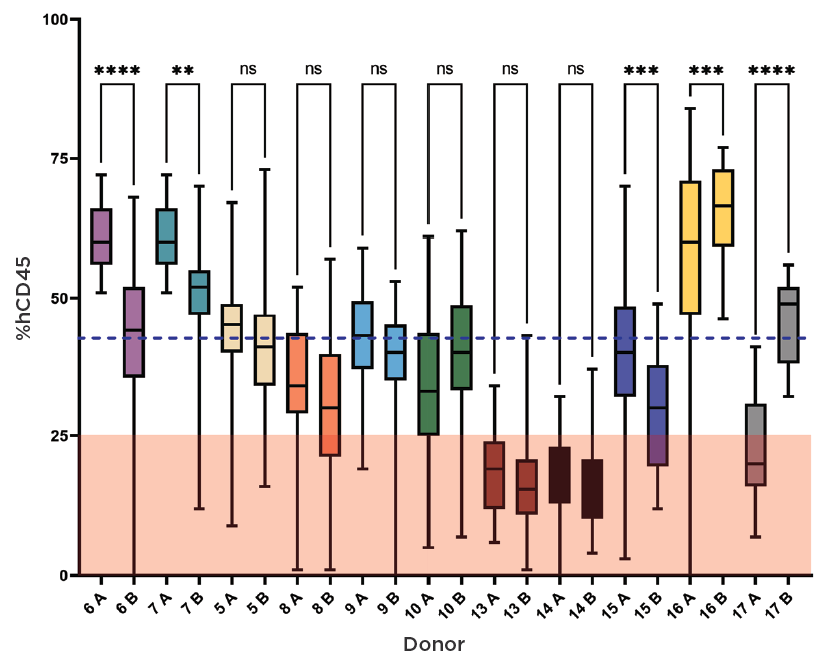
Figure 5: HSCs from the same donor were engrafted twice, on separate days (A and B), to generate two lots of huNOG-EXL mice per donor. The ability of a donor to produce lots where most mice met or failed the QC threshold of ≥25% chimerism was replicated between duplicate lots, with one exception in this test set. To make huNOG-EXL EA mice, Taconic chooses donors with proven engraftment success in order to reduce the risk of lot failure.
Enhanced Myeloid Engraftment in Humanized NOG-EXL Mice
Stable engraftment with significant improvement in myeloid lineage reconstitution and overall cellularity
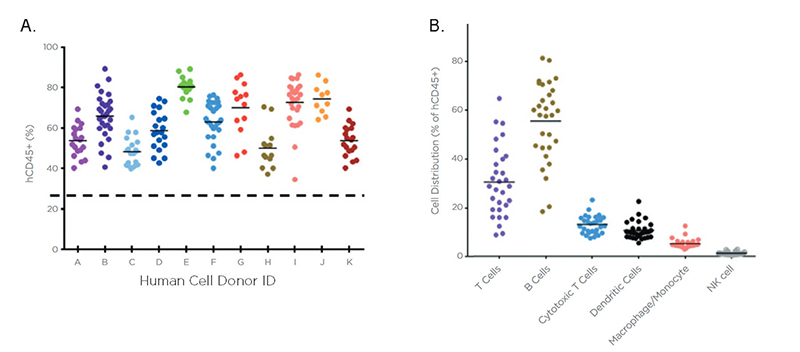
Figure 6: (a) High humanization rate is observed across multiple donors in huNOG-EXL. NOG-EXL (hGM-CSF/hIL-3 NOG) mice were engrafted with human CD34+ hematopoietic stem cells (HSCs) from n=11 different donors and peripheral blood humanizations was monitored by hCD45 antibody. (b) huNOG-EXL mouse supports development of both myeloid and lymphoid cells.
Better engraftment of myeloid populations in huNOG-EXL mice
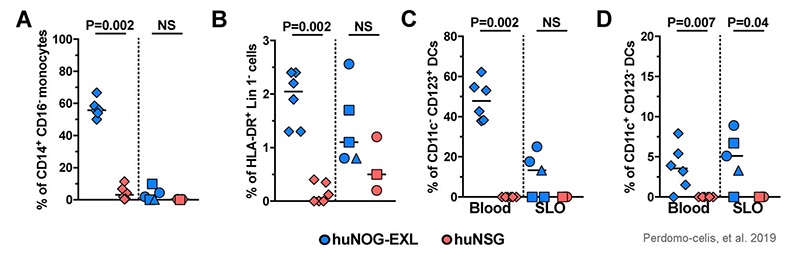
Figure 7: Frequencies of CD14+ CD16- (classical) monocytes from FSC-Ahi CD3- cells (a), HLA-DR+ Lin 1- cells from CD45+ cells (b), CD11c- CD123+ plasmacytoid dendritic cells (c), and CD11c+ CD123- myeloid dendritic cells (d), the latter from HLA-DR+ Lin 1- cells, in blood (diamonds) and secondary lymphoid organs (SLO; spleen: circles; axillary lymph node: squares; mesenteric lymph node: triangles) from huNOG-EXL and huNSG mice. NS: Not statistically significant. Adapted from Perdomo-celis, et al. 2019 under Creative Commons Attribution License.
Human immune cell subsets in huNOG-EXL mice
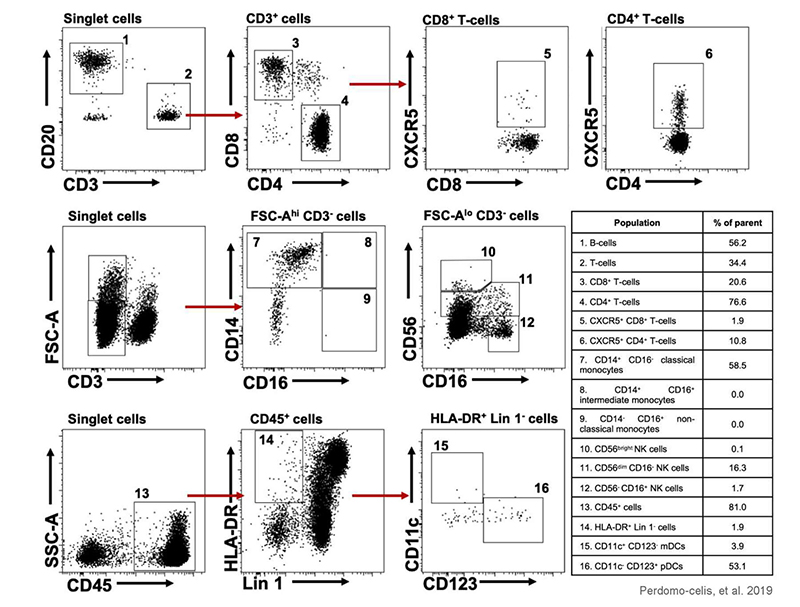
Figure 8: Representative gating strategy from blood cells for the identification of the cell populations evaluated. The number next to the gates represents the respective cell subset found in the adjacent table. Adapted from Perdomo-celis, et al. 2019 under Creative Commons Attribution License.
Tumor Xenograft Models and Immunotherapy Research with huNOG-EXL
Durable anti-tumor response to combination therapy in huNOG-EXL BRCA-deficient tumor model
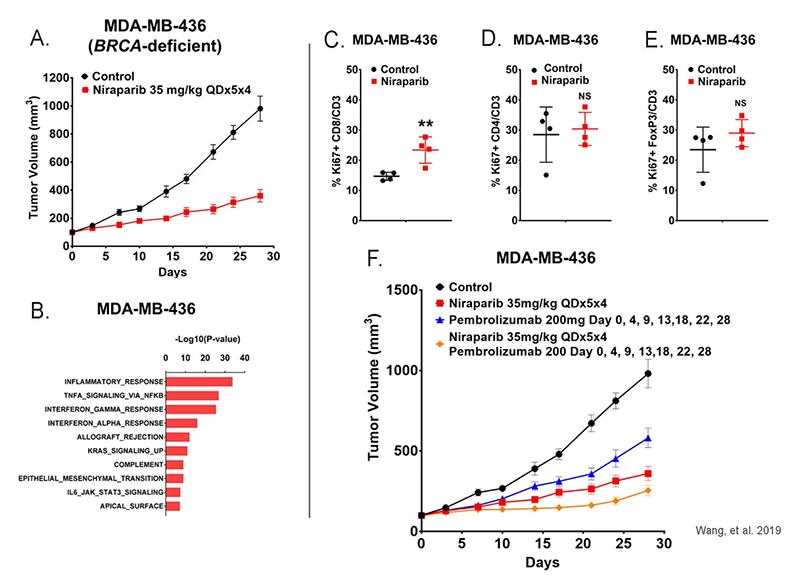
Figure 9: (a) Tumor growth curve for the MDA-MB-436 NOG-EXL humanized model treated with control or 35 mg/kg niraparib daily for 5 days on and 2 days off for 4 weeks (QD × 5 × 4). (b) Significantly upregulated genes identified with a two-sample t test (p <=0.05, fold change >=1.5) were subjected to enrichment analysis of pathway gene sets and demonstrated a significant enrichment of interferon gamma signature and interferon alpha signature genes in niraparib-treated samples. (c-e) Niraparib promoted tumor immune cell infiltration in BRCA-deficient MDA-MB-436 huNOG-EXL humanized tumor model. (f) Tumor growth in the BRCA-deficient MDA-MB-436 model in huNOG-EXL mice treated with 200 mg anti-PD-1 (pembrolizumab) on days 0, 4, 9, 13, 18, 22, and 28; 35 mg/kg niraparib daily for 5 days on and 2 days off for 4 weeks; and the combination of these agents. Adapted from Wang, et al. 2019 under Creative Commons Attribution License.
Immunophenotyping and anti-CTLA4 human immune response in huNOG-EXL mice
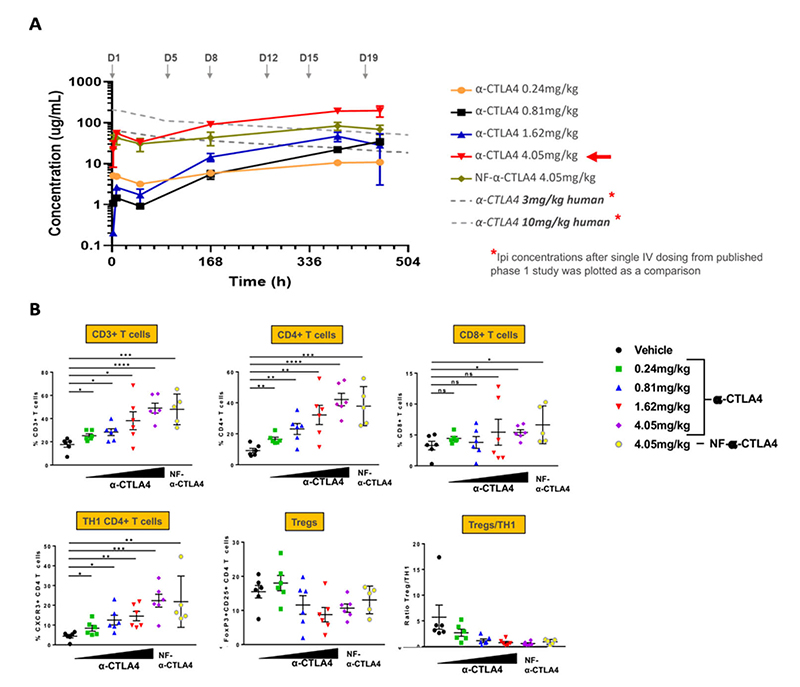
Figure 10: (a) Anti-CTLA4 antibody pharmacokinetics in huNOG-EXL mice. (b) Splenic immunophenotyping of huNOG-EXL mice treated with anti-CLTA4 antibodies. NF: nonfucosylated. Data provided by Dr. Kathryn Fraser, Takeda.
Infectious Disease Research with huNOG-EXL
HIV infection in huNOG-EXL mice affects the frequencies of human immune cells
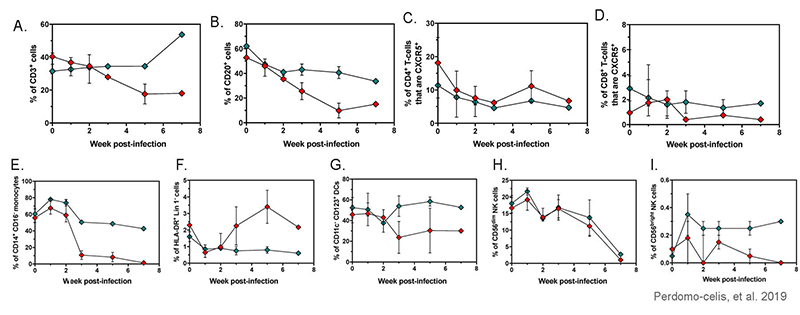
Figure 11: Frequencies of CD3+ cells (a), CD20+ cells (b), CXCR5+ CD4+ T-cells (c), CXCR5+ CD8+ T-cells (d), CD14+ CD16- (classical) monocytes from FSC-Ahi CD3- cells (e), HLA-DR+ Lin 1- cells from CD45+ cells (f), CD11c- CD123+ plasmacytoid dendritic cells from HLA-DR+ Lin 1- cells (g), CD56dim (h), and CD56bright NK cells (i) in huNOG-EXL mice after infection with HIV. Adapted from Perdomo-celis, et al. 2019 under Creative Commons Attribution License.
- Licensing
- Pricing - USD
- Pricing - EUR
- Pricing - DKK
- Pricing - USD Nonprofit
- Pricing - EUR Nonprofit
- Pricing - DKK Nonprofit
- Select my Health Standard
- Get Custom Pricing Guide
huNOG-EXL SA (Standard Access)
Nonprofit users (excluding users at nonprofit foundations which are affiliated with a for-profit entity): For internal research purposes, the CIEA NOG mouse® Conditions of Use for nonprofit users apply. If you wish to perform sponsored research or fee-for-service contract research using the CIEA NOG mouse®, please inquire for access conditions.
For-profit users and users at foundations which are affiliated with for-profit entities: The CIEA NOG mouse® Conditions of Use for for-profit users apply.
The CIEA NOG mouse® is produced and distributed under license rights to the following patents and trademarks:
- Japanese Patent No. 3,753,321
- US Patent No. 7,145,055; 5,464,764; 5,487,992; 5,627,059; 5,631,153; 5,789,215; 6,204,061; 6,653,113; 6,689,610
EP Patent No. 1,338,198 - Japanese Trademark Reg. No. 4,823,423
- US Trademark Reg. No. 3,118,040
- EU Trademark Reg. No. 3,736,758
Pricing - USD
Opportunist Free (OF) Health Standard
HSCCB-13395 Female
HSCCB-13395-F Genotype sp/sp;ko/ko;tg/wt
Available now
| Age in Weeks Post Engraftment | Quantity 1 - 999 |
|---|---|
| 10 to 24 | US$1,558.00 |
HSCCB-13395-PREQC-F Genotype sp/sp;ko/ko;tg/wt
Cohorts are reserved upon order placement and will take 2-4 weeks to fulfill. An estimated lead time will be provided to you within 2-3 business days.
| Age in Weeks Post Engraftment | Quantity 1 - 999 |
|---|---|
| 3 to 9 | US$1,236.00 |
Pricing - EUR
Opportunist Free (OF) Health Standard
HSCCB-13395 Female
HSCCB-13395-F Genotype sp/sp;ko/ko;tg/wt
Available now
| Age in Weeks Post Engraftment | Quantity 1 - 999 |
|---|---|
| 10 to 24 | 1.418,00 € |
HSCCB-13395-PREQC-F Genotype sp/sp;ko/ko;tg/wt
Cohorts are reserved upon order placement and will take 2-4 weeks to fulfill. An estimated lead time will be provided to you within 2-3 business days.
| Age in Weeks Post Engraftment | Quantity 1 - 999 |
|---|---|
| 3 to 9 | 1.125,00 € |
Pricing - DKK
Opportunist Free (OF) Health Standard
HSCCB-13395 Female
HSCCB-13395-F Genotype sp/sp;ko/ko;tg/wt
Available now
| Age in Weeks Post Engraftment | Quantity 1 - 999 |
|---|---|
| 10 to 24 | kr.10.545,00 |
HSCCB-13395-PREQC-F Genotype sp/sp;ko/ko;tg/wt
Cohorts are reserved upon order placement and will take 2-4 weeks to fulfill. An estimated lead time will be provided to you within 2-3 business days.
| Age in Weeks Post Engraftment | Quantity 1 - 999 |
|---|---|
| 3 to 9 | kr.8.368,00 |
Pricing - USD Nonprofit
Opportunist Free (OF) Health Standard
HSCCB-13395 Female
HSCCB-13395-F Genotype sp/sp;ko/ko;tg/wt
Available now
| Age in Weeks Post Engraftment | Quantity 1 - 999 |
|---|---|
| 10 to 24 | US$1,368.00 |
HSCCB-13395-PREQC-F Genotype sp/sp;ko/ko;tg/wt
Cohorts are reserved upon order placement and will take 2-4 weeks to fulfill. An estimated lead time will be provided to you within 2-3 business days.
| Age in Weeks Post Engraftment | Quantity 1 - 999 |
|---|---|
| 3 to 9 | US$1,030.00 |
Pricing - EUR Nonprofit
Opportunist Free (OF) Health Standard
HSCCB-13395 Female
HSCCB-13395-F Genotype sp/sp;ko/ko;tg/wt
Available now
| Age in Weeks Post Engraftment | Quantity 1 - 999 |
|---|---|
| 10 to 24 | 1.245,00 € |
HSCCB-13395-PREQC-F Genotype sp/sp;ko/ko;tg/wt
Cohorts are reserved upon order placement and will take 2-4 weeks to fulfill. An estimated lead time will be provided to you within 2-3 business days.
| Age in Weeks Post Engraftment | Quantity 1 - 999 |
|---|---|
| 3 to 9 | 938,00 € |
Pricing - DKK Nonprofit
Opportunist Free (OF) Health Standard
HSCCB-13395 Female
HSCCB-13395-F Genotype sp/sp;ko/ko;tg/wt
Available now
| Age in Weeks Post Engraftment | Quantity 1 - 999 |
|---|---|
| 10 to 24 | kr.9.258,00 |
HSCCB-13395-PREQC-F Genotype sp/sp;ko/ko;tg/wt
Cohorts are reserved upon order placement and will take 2-4 weeks to fulfill. An estimated lead time will be provided to you within 2-3 business days.
| Age in Weeks Post Engraftment | Quantity 1 - 999 |
|---|---|
| 3 to 9 | kr.6.974,00 |
Select my Health Standard
Need help choosing the right Taconic Biosciences health standard for your research?
Use the Health Standard Selector to enter your exclusion list. The tool will tell you which health standards meet your requirements.
Get custom pricing guide
Schedule A Scientific Consultation
Speak with a PhD-level Field Application Scientist who can help you select the most appropriate model and maximize your experimental success.
















.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)


.jpg)
.jpg)




.jpg)





.jpg)

.jpg)









