 The recent emergence of the novel, pathogenic SARS-coronavirus 2 (SARS-CoV-2, COVID-19), and its rapid international spread pose a global health emergency. The SARS-CoV-2 virus belongs to the family of coronaviridae viruses, which are enveloped, positive-sense, single-stranded RNA viruses. Entry into human (mammalian) cells depends on binding of the viral spike (S) proteins to human cellular receptors and on S protein priming by host cell proteases. Patients with COVID-19 present with many symptoms and predominantly suffer from respiratory and pulmonary complications1. A serious complication of COVID-19, and especially amongst a large percentage of COVID-19 deaths, is acute respiratory distress syndrome (ARDS)2. This is the most severe form of clinical acute lung injury and has a very high mortality rate (30-60%)3. Increasing publications reveal that SARS-CoV-2 uses the SARS-CoV receptor ACE2 for cellular entry and the serine protease TMPRSS2 for S protein priming4-6.
The recent emergence of the novel, pathogenic SARS-coronavirus 2 (SARS-CoV-2, COVID-19), and its rapid international spread pose a global health emergency. The SARS-CoV-2 virus belongs to the family of coronaviridae viruses, which are enveloped, positive-sense, single-stranded RNA viruses. Entry into human (mammalian) cells depends on binding of the viral spike (S) proteins to human cellular receptors and on S protein priming by host cell proteases. Patients with COVID-19 present with many symptoms and predominantly suffer from respiratory and pulmonary complications1. A serious complication of COVID-19, and especially amongst a large percentage of COVID-19 deaths, is acute respiratory distress syndrome (ARDS)2. This is the most severe form of clinical acute lung injury and has a very high mortality rate (30-60%)3. Increasing publications reveal that SARS-CoV-2 uses the SARS-CoV receptor ACE2 for cellular entry and the serine protease TMPRSS2 for S protein priming4-6. - Mouse-adapted SARS-CoV-2 strains unlock broader mouse modeling of COVID-19
- Transgenic hACE2 mice for SARS-CoV-2 and COVID-19
- Aged Inbred Mice May Advance Research Into Age-Related Mortality in Coronavirus Infections
- An Overview of Mouse Models for COVID-19
- Safety Testing Critical for COVID-19 Therapies and Vaccines
Genetically Engineered Mouse Models for SARS Research
Genetically engineered mouse models (GEMs) have been instructive for the development of novel treatments and vaccines for SARS and are also expected to be valuable in researching COVID-19 treatments. GEM knockout (KO) mouse models of angiotensin-converting enzyme (Ace2)7,8 and transmembrane protease serine-type 2 (Tmprss2)9 were instrumental in demonstrating the critical roles that these proteins play in SARS infection and in the development of pulmonary symptoms. The SARS S protein binds to the ACE2 receptor, which is further primed by TMPRSS2 within human cells10,11. Ace2 Knockout mice have been critical in SARS and ARDS research in demonstrating that the binding of SARS viral S protein to ACE2 in mice down-regulates ACE2 expression and that the loss of ACE2 expression is associated with severe lung failure12. Likewise, Tmprss2 KO mice have been utilized in SARS research to demonstrate the involvement of Tmprss2 in SARS-CoV entry into cells, and its inhibition may constitute a treatment/prophylaxis mechanism. Emerging literature supports the utility of these Ace2 and Tmprss2 GEM models for contemporaneous studies into COVID-19 disease pathogenesis and therapeutics 13,14.The Angiotensin-Converting Enzyme 2 (ACE2) Knockout Mouse
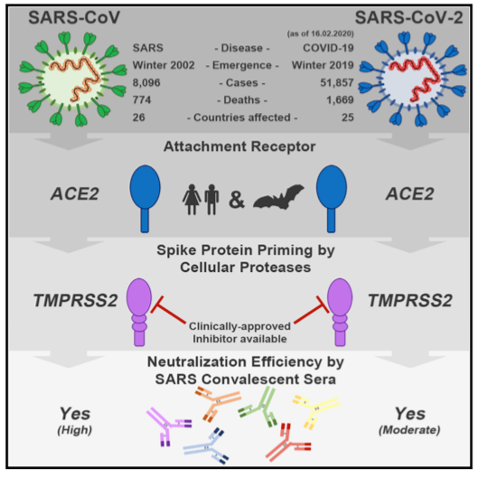
Figure Legend: Graphical abstract illustrating how the S protein of the SARS-CoV-2 virus binds to the SARS-CoV receptor ACE2 in order to enter human host cells, which is followed by priming by TMPRSS2. Therefore, antibodies against the SARS-CoV S protein or inhibitors of TMPRSS2 may offer protection against COVID-19 pathologies4.
The Ace2 KO mouse in lung disease research
The role of ACE2 in the pathogenesis of acute lung injury such as ARDS was demonstrated by utilizing Ace2 KO and wild type (WT) mice in experimental models (namely acid-aspiration-induced ARDS, endotoxin-induced ARDS, and peritoneal sepsis-induced ARDS) that mimic the common lung pathology observed in several human diseases. Ace2 KO mice demonstrated severe disease compared to WT mice, with enhanced vascular permeability, increased lung edema, neutrophil accumulation, and worsened lung function8. These symptoms of acute lung injury were improved by treatment with catalytically active recombinant ACE2 protein in both WT and Ace2 KO mice. These results show that the loss of ACE2 is essential for disease pathogenesis, demonstrating a critical protective role of ACE2 in acute lung injury7.The Ace2 KO Mouse in Studies of Infectious Respiratory Disease and SARS
The ACE2 protein has been demonstrated to be an essential receptor for SARS infections in vivo by binding to the SARS-CoV S protein, which promotes syncytia formation in the lungs of diseased humans12,16, in mice in vivo8, and similarly for SARS-CoV-2 in vitro4-6. The Ace2 KO mouse was used in a mouse-adapted SARS-CoV infection model and found to be resistant to viral infection12. No lung histology from Ace2 KO mice challenged with SARS-CoV showed signs of inflammation12, whereas some (but not all) SARS-infected WT mice displayed mild inflammation with leukocyte infiltration8,17,18. These results and additional publications demonstrated the validity of Ace2 KO mouse in evaluating a recombinant ACE2 protein as a treatment to block the spread of SARS and to protect SARS patients from developing lung failure7,8.Thus the Ace2 KO mouse can be used:
- to investigate the pathophysiological role of ACE2 in SARS infections and other emerging infectious diseases that affect the lungs
- to study the role of acute lung injury such as ARDS
- to interrogate the relevance of ACE2 as a target for therapeutic intervention
The Transmembrane Protease Serine Type 2 (TMPRSS2) Knockout Mouse
Mice genetically deficient for Transmembrane Protease Serine Type 2 (Tmprss2) have been used to study the role of TMPRSS2 during coronavirus infection in vivo9. The human TMPRSS2 gene encodes for an androgen-regulated type II transmembrane serine protease (TTSP), which is highly expressed in normal human prostate epithelium and has been implicated in prostate carcinogenesis 19. TMPRSS2 (also known as epitheliasin) is also highly expressed in the epithelial tissues, including those lining the upper respiratory airways, bronchi, and lungs in humans20. There is related interest in TMPRSS2 is due to its ability to cleave coronavirus spike proteins to induce virus-cell membrane fusion at the cell surface and facilitate rapid early viral entry into the host cell21-23. Thus, substrate analogue inhibitors of TMPRSS2's active sites24,25 are increasingly investigated as potential therapeutic targets for coronaviral infections as well as influenza viral infections10,13,26.The role of Tmprss2 KO mouse in SARS research
The Tmprss2 KO mouse was used in a mouse-adapted SARS-CoV infection model and exhibited no loss of body weight and weak pro-inflammatory responses upon viral infection when compared to identically infected WT mice that developed signs of weight loss and acute pneumonia9. The loss of Tmprss2 restricted the primary sites of viral infection within the lungs, diminished viral replication (especially within the bronchioles), viral dissemination within the respiratory airways, and was accompanied by less severe immunopathology in the Tmprss2 KO as compared to the WT mouse. However, the Tmprss2 KO mouse exhibited weakened or delayed inflammatory chemokine and cytokine responses that are mediated by Toll-like receptor 3 (TLR3) when intra-nasally stimulated with poly(I·C), which is a synthetic analogue of double-stranded RNA in order to assess the effect of Tmprss2 on the innate immune responses9. These results demonstrate that Tmprss2 plays a crucial role in viral spread within the airway of murine models infected by SARS-CoV and in the resulting pulmonary pathology and systemic immunopathology. Additional findings suggest that Tmprss2 may contribute to more severe or rapid immunopathology in WT mice by increasing the levels of inflammatory cytokines and chemokines after TLR3 stimulation.Thus Tmprss2 KO mouse can be used in the research of SARS and COVID-19 infections:10,13,26
- to investigate the pathophysiological role of TMPRSS2
- to interrogate TMPRSS2 as a potential therapeutic target
















.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)


.jpg)
.jpg)




.jpg)





.jpg)

.jpg)




