The interaction between chemokines and their receptors on immune cells is required for immune cells to move throughout the body and into peripheral tissues. Where these human chemokines come from and their levels in humanized immune system (HIS) mice has, until recently, been an unanswered question.
HIS mice include
NOG,
NOG-EXL, and NSG-SGM3 mice that have been engrafted with human CD34+ hematopoietic stem cells (HSCs). In a recent study by Maser et al., the authors performed a head-to-head comparison of humanized NOG, NOG-EXL, and NSG-SGM3 to understand how the composition of immune cells and related chemokines compares between the three. Each of these strains supports different subsets of human immune cell populations. In particular, humanized NOG mice support abundant functional human T cells, while humanized NOG-EXL and humanized NSG-SGM3 additionally support various myeloid cell subsets.
The authors noticed differences between HSC-engrafted NOG-EXL and HSC-engrafted NSG-SGM3 — the two strains that support myeloid cells — in both the dominant subsets of myeloid cells and the cytokine levels. The significance of this publication lies in the fact that choosing the appropriate HIS model for your study includes selecting one that supports your cell type of interest. How might the differences in myeloid cell subpopulations and chemokine levels between HSC-engrafted NOG, HSC-engrafted NOG-EXL, and HSC-engrafted NSG-SGM3 impact your decision? Let's explore the biology of key myeloid-associated chemokines detected in the serum of these mice and their role in myeloid cell biology.
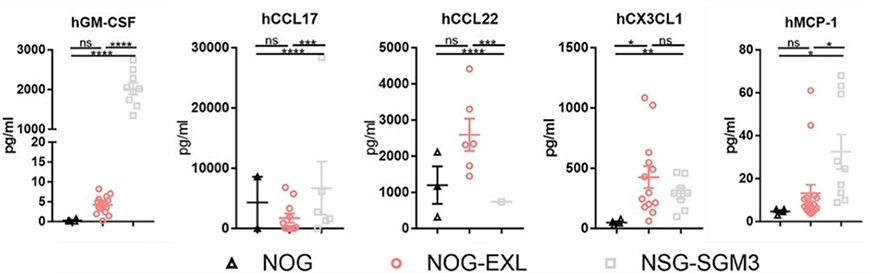
Figure 1: Myeloid cell-associated chemokines measured in the serum of various strains of humanized immune system mice. Adapted from Maser et al1.
The intertwined roles of CX3CL1/fractalkine and CCL2/CCL2 biology
Among the chemokines evaluated in the Maser study were CX3CL1 (also known as fractalkine), which engages with its receptor CX3CR1, and MCP-1 (also known as CCL2), whose receptor is CCR2. The receptor CX3CR1 is present on immune cells, while fractalkine is produced by activated endothelial cells, macrophages, dendritic cells, and other tissues
2-4. The distribution of CCR2 expression is similar, with expression on monocytes and other myeloid cells, T cells, B cells, and NK cells
5. CCL2 is produced by many cells under inflammatory conditions: this includes dendritic cells, macrophages, monocytes, neutrophils, and eosinophils, as well as non-immune cells including epithelial and smooth muscle cells
6-8.
In the context of myeloid cell development, the fractalkine/CX3CR1 axis first plays a role during monocyte development in the bone marrow, as CX3CR1 levels increase on monocytes as they mature in the bone marrow
9. In this context, CX3CR1 expression is a marker of commitment to the monocyte/macrophage/dendritic cell lineage
10. The engagement of CX3CR1 by fractalkine is responsible for the recruitment of CD16+ monocytes into peripheral/inflamed tissue
11,12. The role of CX3CL1 on cells of monocytic lineage is counterbalanced by that of CCL2, as inflammatory monocytes express high CCR2 whereas tissue-resident monocytes express high CXCR1
13. The role of CCR2/CCL2 signaling on myeloid cells is quite broad. It impacts a variety of cellular processes: chemotaxis, macrophage phagocytosis, monocyte degranulation, myeloid-derived suppressor cell (MDSC)-mediated immunosuppression, and both M1 and M2 macrophage polarization
14.
HIS mice are frequently used in immuno-oncology studies, and CX3CL1 plays a role in the trafficking of monocytes into the tumor microenvironment. There is evidence that it can play a role in either promoting or suppressing tumor growth, depending on other pro- or anti-inflammatory factors within the tumor microenvironment
15. CCL2/CCR2 signaling, on the other hand, is typically associated with a poor prognosis in the cancer setting
16.
The direct comparison of chemokine levels in the Maser study noted that both HSC-engrafted NOG-EXL and HSC-engrafted NSG-SGM3 mice expressed similar levels of CX3CL1, while HSC-engrafted NOG mice expressed significantly lower levels of this chemokine. The levels of CCL2, however, did differ between these strains, with HSC-engrafted NSG-SGM3 mice expressing significantly higher levels of the chemokine than HSC-engrafted NOG-EXL; there was no significant difference in its expression between HSC-engrafted NOG and HSC-engrafted NOG-EXL mice. What might be the impact of CXC3L1 and CCL2 expression in humanized immune system mice? Although no studies have directly investigated this so far, one might hypothesize that the presence of CX3CL1 supports myeloid cell function in both models, while the higher CCL2 expression in HSC-engrafted NSG-SGM3 over HSC-engrafted NOG-EXL contributes to the more inflammatory profile in that strain.
The immunosuppressive chemokines CCL17 and CCL22
CCL17 and CCL22, both also evaluated in the study by Maser et al., signal through a common receptor, CCR4. CCR4 is expressed on Type-2 T helper (Th2) cells and regulatory T cells (Tregs)
17,18. CCL17 is expressed by dendritic cells in peripheral lymph nodes and barrier tissues, where they stimulate T cell responses in the periphery, including the skin and mucosa
19. The expression of CCL22 is induced in dendritic cells, monocytes, and macrophages in inflammatory environments
20,21. These chemokines as well as CX3CL1 are clustered together in the human genome
22. However, CCL17 and CCL22 signals are typically associated with attenuation of an immune response due to their signaling on Th2 and Treg cells.
A feed-forward loop via T cell-derived GM-CSF promotes CCL22 secretion by dendritic cells
23. GM-CSF can also induce the production of CCL17 from monocytes
24. It may not come as a surprise that both CCL22 and CCL17, whose production by myeloid cells is induced by GM-CSF, has significantly higher expression in mice expressing transgenic hGM-CSF, i.e., the HSC-engrafted NOG-EXL and HSC-engrafted NSG-SGM3, compared to HSC-engrafted NOG. Importantly, the study by Maser et al. also looked at the level of GM-CSF in human tumor xenografts implanted into HSC-engrafted NOG and HSC-engrafted NOG-EXL mice. They showed that the tumor lysates isolated from these mice expressed varying levels of GM-CSF that correlated with the specific tumor xenograft being evaluated rather than the background strain, despite the serum levels of these chemokines correlating to strain. The choice of both mouse model and tumor xenograft model can therefore impact your study outcome.
In the context of immuno-oncology, because CCR4 is expressed on regulatory T cells, this pathway is an emerging target for immunotherapy
25. In the tumor microenvironment, CCL17 and CCL22 can be produced by tumor-associated M2 macrophages and dendritic cells, and these chemokines attract Tregs into the tumor microenvironment
26,27. Indeed, tumors with high levels of CCL17 and CCL22 expression have concomitant high infiltration of Tregs
28. Reducing the intratumoral burden of CCL17 or CCL22 is another immunotherapeutic approach to reduce the recruitment of Tregs into the tumor microenvironment. For example, tumor-infiltrating dendritic cells express CCL22 following stimulation by (tumor-derived) IL-1alpha and this can be blocked by treatment with an IL-1 receptor antagonist
29.
Incorporating chemokine data into selection of a mouse model for immuno-oncology research
The chemokines summarized here are not the only chemokines that can be produced by human immune cells in HIS mice. However, CX3CL1, CCL2, CCL17, and CCL22 are all important chemokines produced by myeloid cells that are also important in immuno-oncology. Not only does it remain important to select a mouse model based on the cell types of interest in your preclinical study, but keep in mind the chemokines found in HIS models such as HSC-engrafted NOG (such as Taconic Biosciences'
huNOG model) versus HSC-engrafted NOG-EXL (including Taconic's
huNOG-EXL) can also impact your experimental results.
 Watch the Taconic Biosciences Webinar:
Watch the Taconic Biosciences Webinar:
References:
1. Maser, I.-P.; Hoves, S.; Bayer, C.; Heidkamp, G.; Nimmerjahn, F.; Eckmann, J.; Ries, C. H. The Tumor Milieu Promotes Functional Human Tumor-Resident Plasmacytoid Dendritic Cells in Humanized Mouse Models. Front. Immunol. 2020, 11.
2. Bazan, J. F.; Bacon, K. B.; Hardiman, G.; Wang, W.; Soo, K.; Rossi, D.; Greaves, D. R.; Zlotnik, A.; Schall, T. J. A New Class of Membrane-Bound Chemokine with a CX3C Motif. Nature 1997, 385 (6617), 640-644.
3. Greaves, D. R.; Häkkinen, T.; Lucas, A. D.; Liddiard, K.; Jones, E.; Quinn, C. M.; Senaratne, J.; Green, F. R.; Tyson, K.; Boyle, J.; Shanahan, C.; Weissberg, P. L.; Gordon, S.; Ylä-Hertualla, S. Linked Chromosome 16q13 Chemokines, Macrophage-Derived Chemokine, Fractalkine, and Thymus- and Activation-Regulated Chemokine, Are Expressed in Human Atherosclerotic Lesions. ATVB 2001, 21 (6), 923-929.
4. Papadopoulos, E. J.; Sassetti, C.; Saeki, H.; Yamada, N.; Kawamura, T.; Fitzhugh, D. J.; Saraf, M. A.; Schall, T.; Blauvelt, A.; Rosen, S. D.; Hwang, S. T. Fractalkine, a CX3C Chemokine, Is Expressed by Dendritic Cells and Is up-Regulated upon Dendritic Cell Maturation. Eur. J. Immunol. 1999, 29 (8), 2551-2559.
5. Frade, J. M.; Mellado, M.; del Real, G.; Gutierrez-Ramos, J. C.; Lind, P.; Martinez-A, C. Characterization of the CCR2 chemokine receptor: functional CCR2 receptor expression in B cells. J. Immunol. 1997, 159(11), 5576-5584.
6. Gschwandtner, M.; Derler, R.; Midwood, K. S. More Than Just Attractive: How CCL2 Influences Myeloid Cell Behavior Beyond Chemotaxis. Front. Immunol. 2019, 10.
7. Standiford, T. J.; Kunkel, S. L.; Phan, S. H.; Rollins, B. J.; Strieter, R. M. Alveolar Macrophage-Derived Cytokines Induce Monocyte Chemoattractant Protein-1 Expression from Human Pulmonary Type II-like Epithelial Cells. Journal of Biological Chemistry 1991, 266 (15), 9912-9918.
8. Brown, Z.; Strieter, R. M.; Neild, G. H.; Thompson, R. C.; Kunkel, S. L.; Westwick, J. IL-1 Receptor Antagonist Inhibits Monocyte Chemotactic Peptide 1 Generation by Human Mesangial Cells. Kidney International 1992, 42 (1), 95-101.
9. Jacquelin, S.; Licata, F.; Dorgham, K.; Hermand, P.; Poupel, L.; Guyon, E.; Deterre, P.; Hume, D. A.; Combadière, C.; Boissonnas, A. CX3CR1 Reduces Ly6Chigh-Monocyte Motility within and Release from the Bone Marrow after Chemotherapy in Mice. Blood 2013, 122 (5), 674-683.
10. Auffray, C.; Fogg, D. K.; Narni-Mancinelli, E.; Senechal, B.; Trouillet, C.; Saederup, N.; Leemput, J.; Bigot, K.; Campisi, L.; Abitbol, M.; Molina, T.; Charo, I.; Hume, D. A.; Cumano, A.; Lauvau, G.; Geissmann, F. CX3CR1+ CD115+ CD135+ Common Macrophage/DC Precursors and the Role of CX3CR1 in Their Response to Inflammation. Journal of Experimental Medicine 2009, 206 (3), 595-606.
11. Auffray, C.; Fogg, D.; Garfa, M.; Elain, G.; Join-Lambert, O.; Kayal, S.; Sarnacki, S.; Cumano, A.; Lauvau, G.; Geissmann, F. Monitoring of Blood Vessels and Tissues by a Population of Monocytes with Patrolling Behavior. Science 2007, 317 (5838), 666-670.
12. Ancuta, P.; Rao, R.; Moses, A.; Mehle, A.; Shaw, S. K.; Luscinskas, F. W.; Gabuzda, D. Fractalkine Preferentially Mediates Arrest and Migration of CD16+ Monocytes. Journal of Experimental Medicine 2003, 197 (12), 1701-1707.
13. Geissmann, F.; Jung, S.; Littman, D. R. Blood Monocytes Consist of Two Principal Subsets with Distinct Migratory Properties. Immunity 2003, 19 (1), 71-82.
14. Gschwandtner, M.; Derler, R.; Midwood, K. S. More Than Just Attractive: How CCL2 Influences Myeloid Cell Behavior Beyond Chemotaxis. Front. Immunol. 2019, 10.
15. Sidibe, A.; Ropraz, P.; Jemelin, S.; Emre, Y.; Poittevin, M.; Pocard, M.; Bradfield, P. F.; Imhof, B. A. Angiogenic Factor-Driven Inflammation Promotes Extravasation of Human Proangiogenic Monocytes to Tumours. Nat Commun 2018, 9 (1).
16. Hao, Q.; Vadgama, J. V.; Wang, P. CCL2/CCR2 Signaling in Cancer Pathogenesis. Cell Commun Signal 2020, 18 (1).
17. Andrew, D. P., Chang, M.-shi, McNinch, J., Wathen, S. T., Rihanek, M., Tseng, J., Spellberg, J. P., & Elias, C. G. STCP-1 (MDC) cc chemokine acts specifically on chronically activated th2 lymphocytes and is produced by monocytes on stimulation with th2 cytokines IL-4 and IL-13. The Journal of Immunology 161 (9) 5027-5038.
18. Iellem, A.; Mariani, M.; Lang, R.; Recalde, H.; Panina-Bordignon, P.; Sinigaglia, F.; D'Ambrosio, D. Unique Chemotactic Response Profile and Specific Expression of Chemokine Receptors Ccr4 and Ccr8 by Cd4+Cd25+ Regulatory T Cells. Journal of Experimental Medicine, 2001, 194, 847-854.
19. Alferink, J.; Lieberam, I.; Reindl, W.; Behrens, A.; Weiß, S.; Hüser, N.; Gerauer, K.; Ross, R.; Reske-Kunz, A. B.; Ahmad-Nejad, P.; Wagner, H.; Förster, I. Compartmentalized Production of CCL17 In Vivo. Journal of Experimental Medicine, 2003, 197, 585-599.
20. Tang, H. L.; Cyster, J. G. Chemokine Up-Regulation and Activated T Cell Attraction by Maturing Dendritic Cells. Science, 1999, 284, 819-822.
21. Kimura, S.; Tanimoto, A.; Wang, K.-Y.; Shimajiri, S.; Guo, X.; Tasaki, T.; Yamada, S.; Sasaguri, Y. Expression of Macrophage-Derived Chemokine (CCL22) in Atherosclerosis and Regulation by Histamine via the H2 Receptor. Pathology International, 2012, 62, 675-683.
22. Nomiyama, H.; Imai, T.; Kusuda, J.; Miura, R.; Callen, D. F.; Yoshie, O. Human Chemokines Fractalkine (SCYD1), MDC (SCYA22) and TARC (SCYA17) Are Clustered on Chromosome 16q13. Cytogenetic and Genome Research, 1998, 81, 10-11.
23. Piseddu, I.; Röhrle, N.; Knott, M. M. L.; Moder, S.; Eiber, S.; Schnell, K.; Vetter, V.; Meyer, B.; Layritz, P.; Kühnemuth, B.; Wiedemann, G. M.; Gruen, J.; Perleberg, C.; Rapp, M.; Endres, S.; Anz, D. Constitutive Expression of CCL22 Is Mediated by T Cell-Derived GM-CSF. The Journal of Immunology, 2020, 205, 2056-2065.
24. Achuthan, A.; Cook, A. D.; Lee, M.-C.; Saleh, R.; Khiew, H.-W.; Chang, M. W. N.; Louis, C.; Fleetwood, A. J.; Lacey, D. C.; Christensen, A. D.; Frye, A. T.; Lam, P. Y.; Kusano, H.; Nomura, K.; Steiner, N.; Förster, I.; Nutt, S. L.; Olshansky, M.; Turner, S. J.; Hamilton, J. A. Granulocyte Macrophage Colony-Stimulating Factor Induces CCL17 Production via IRF4 to Mediate Inflammation. Journal of Clinical Investigation, 2016, 126, 3453-3466.
25. Marshall, L. A.; Marubayashi, S.; Jorapur, A.; Jacobson, S.; Zibinsky, M.; Robles, O.; Hu, D. X.; Jackson, J. J.; Pookot, D.; Sanchez, J.; Brovarney, M.; Wadsworth, A.; Chian, D.; Wustrow, D.; Kassner, P. D.; Cutler, G.; Wong, B.; Brockstedt, D. G.; Talay, O. Tumors Establish Resistance to Immunotherapy by Regulating Treg Recruitment via CCR4. Journal for ImmunoTherapy of Cancer, 2020, 8, e000764.
26. Furudate, S.; Fujimura, T.; Kakizaki, A.; Kambayashi, Y.; Asano, M.; Watabe, A.; Aiba, S. The Possible Interaction between Periostin Expressed by Cancer Stroma and Tumor-Associated Macrophages in Developing Mycosis Fungoides. Experimental Dermatology, 2015, 25, 107-112.
27. Wiedemann, G. M.; Knott, M. M. L.; Vetter, V. K.; Rapp, M.; Haubner, S.; Fesseler, J.; Kühnemuth, B.; Layritz, P.; Thaler, R.; Kruger, S.; Ormanns, S.; Mayr, D.; Endres, S.; Anz, D. Cancer Cell-Derived IL-1α Induces CCL22 and the Recruitment of Regulatory T Cells. OncoImmunology, 2016, 5, e1175794.
28. Talay, O.; Jorapur, A.; Jacobson, S.; Marubayashi, S.; Marshall, L.; Suthram, S.; Robles, O.; Ketcham, J.; Reilly, M. K.; Younai, A.; Biannic, B.; Hu, D.; Bui, M.; Schwarz, J.; Kassner, P.; Cutler, G. Abstract 4752: EBV Associated Tumors Have Increased Regulatory T Cell Recruitment and Are Therefore a Potential Indication for Treatment with Potent and Selective Small Molecule CCR4 Antagonists. Immunology, 2018.
 The interaction between chemokines and their receptors on immune cells is required for immune cells to move throughout the body and into peripheral tissues. Where these human chemokines come from and their levels in humanized immune system (HIS) mice has, until recently, been an unanswered question.
The interaction between chemokines and their receptors on immune cells is required for immune cells to move throughout the body and into peripheral tissues. Where these human chemokines come from and their levels in humanized immune system (HIS) mice has, until recently, been an unanswered question. 
















.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)


.jpg)
.jpg)




.jpg)





.jpg)

.jpg)




