Application Areas:
huNOG

| Model No. | Nomenclature | Genotype |
|---|---|---|
| HSCCB-NOG-F | NOD.Cg-Prkdcscid Il2rgtm1Sug/ JicTac | sp/sp;ko/ko |
- Description
- Related Products & Services
- Data
- Price & Licensing
- Health Report
- Overview
- Genetics
- Guides & Publications
- Applications & Therapeutic Areas
- Transit, Housing & Welfare
- Diet
Overview
Nomenclature: NOD.Cg-Prkdcscid Il2rgtm1Sug/ JicTac
- Now available from US and EU production sites.
- Human lymphocytes are present in peripheral blood, bone marrow, thymus and spleen.
- All mice shipped meet a QC spec of ≥25% hCD45+ in peripheral blood as assessed at 12 weeks post-engraftment, unless otherwise requested.
- Long term studies are possible in huNOG mice, with literature reports of stable engraftment and hematopoiesis for one year or more. However, not all human immune cell types are present, and some cell types may not be completely functional.
- Humanized immune system mice require special housing and husbandry. We recommend reviewing the the comprehensive document Taconic's Humanized Immune System Models: Licensing, Care & Resources and the Video: Care of HIS mice for experimental success prior to ordering.
Orders by weight: Taconic cannot accept orders by weight for this model. Please note that shipments may contain animals with a larger weight variation.
Availability
- Now available from US and EU production sites.
- Custom options such as HLA selection or cell population are available with additional fee. Inquire regarding your specific needs.
- Orders with a specification regarding number of mice per donor are subject to availability and are not guaranteed, as orders may need to be adjusted at time of packing.
NOTE: Please contact Taconic prior to your first order for a review of unpacking instructions. Tattoo numbers may repeat between engraftment lots. Individual ID number is determined through examination of the tattoo PLUS the box and section location.
A Humanized Immune System (HIS) Mouse with Long-Term Engraftment of Human Lymphoid Cells
Genetics
Applications & Therapeutic Areas
- Immunology
- Infectious Disease
- Oncology & Immuno-Oncology
- Safety Assessment
- Transgenic Model Generation Support
- Cell and Tissue Humanized
- Inflammation
Transit, Housing & Welfare
Need more info? Click the live chat button or Contact Us
Taconic packs humanized immune system mice as cagemates - one engraftment lot per Taconic Transit Cage (TTC). Taconic does not combine different lots into a single TTC.
Diet
- Services
Data
Humanized NOG Mice are Replete with Human B Cells and CD3+ T Cells
Flow cytometry analysis of human cell lineages in huNOG mice
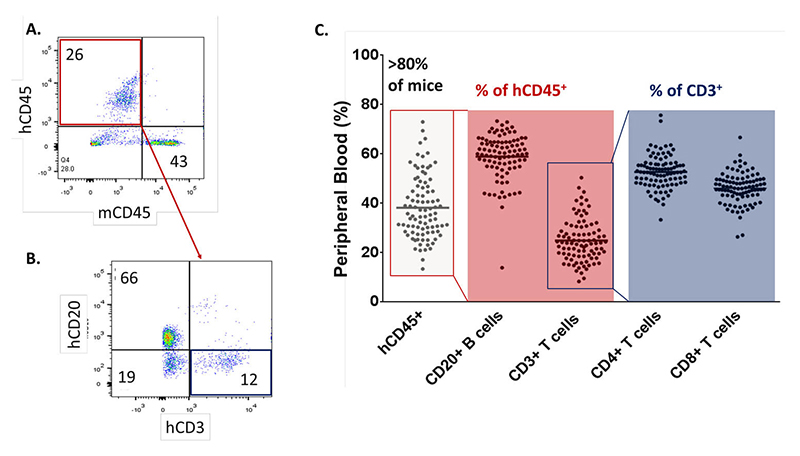
Figure 1: (a) Representative flow cytometry plot of the human (hCD45+) cell to mouse cell (mCD45+) ratio at 12 weeks post engraftment. (b) Relative percentage of human cells expressing the B cell marker CD20 vs the T cell marker CD3. (c) Consistent engraftment ratios of greater than 25% hCD45+ cells in over 80 percent of animals (n=91), with highly reproducible relative T cell and B cell enrichment levels. Each dot represents an individual mouse analyzed by flow cytometry 12 weeks post CD34+ HSC engraftment.
Oncology & Immunotherapy Research with huNOG Mice
Combinatory treatment of oncolytic adenovirus with anti PD-1 shows synergistic anti-tumor effect in huNOG tumor model
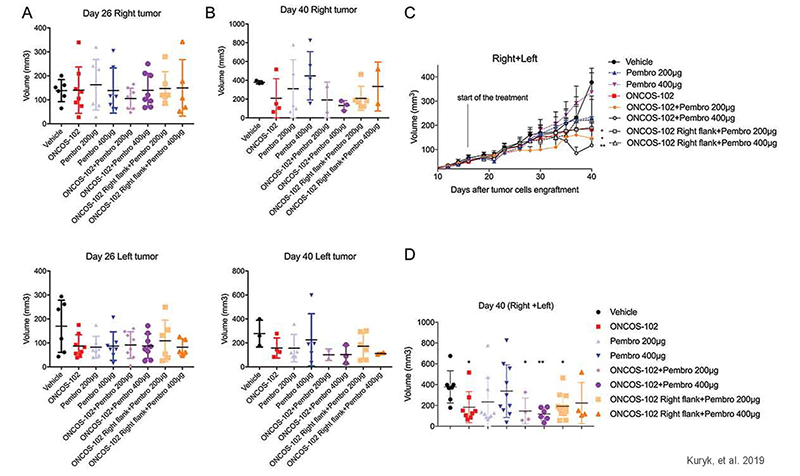
Figure 2: Effects of ONCOS-102 and pembrolizumab treatments on tumor volume of A2058 engrafted huNOG mice. (a) Tumor volume on day 26 for right and left tumor. (b) Tumor volume on day 40 for right and left tumor. (c) Tumor volume throughout the treatment (pooled for right and left tumors). (d) Tumor volume on day 40 (pooled for right and left tumors). Adapted from Kuryk, et al. 2019 under Creative Commons Attribution License.
Human immune system increases breast cancer-induced osteoblastic bone growth in huNOG mice
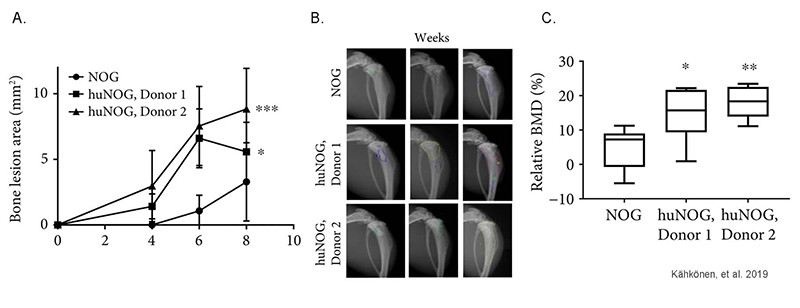
Figure 3: Tumor-induced bone changes in NOG and huNOG mice. (a) Quantification of the bone lesion area from X-ray imaging (b) at 4, 6, and 8 weeks after cancer cell inoculation. (c) BMD in the tumor-bearing tibia relative to BMD in the healthy tibia. Adapted from Kähkönen, et al. 2019 under Creative Commons Attribution License.
- Licensing
- Pricing - USD
- Pricing - EUR
- Pricing - DKK
- Pricing - USD Nonprofit
- Pricing - EUR Nonprofit
- Pricing - DKK Nonprofit
- Select my Health Standard
- Get Custom Pricing Guide
huNOG
Nonprofit users (excluding users at nonprofit foundations which are affiliated with a for-profit entity): For internal research purposes, the CIEA NOG mouse® Conditions of Use for nonprofit users apply. If you wish to perform sponsored research or fee-for-service contract research using the CIEA NOG mouse®, please inquire for access conditions.
For-profit users and users at foundations which are affiliated with for-profit entities: The CIEA NOG mouse® Conditions of Use for for-profit users apply.
The CIEA NOG mouse® is produced and distributed under license rights to the following patents and trademarks:
- Japanese Patent No. 3,753,321
- US Patent No. 7,145,055; 5,464,764; 5,487,992; 5,627,059; 5,631,153; 5,789,215; 6,204,061; 6,653,113; 6,689,610
EP Patent No. 1,338,198 - Japanese Trademark Reg. No. 4,823,423
- US Trademark Reg. No. 3,118,040
- EU Trademark Reg. No. 3,736,758
Pricing - USD
Opportunist Free (OF) Health Standard
HSCCB-NOG Female
HSCCB-NOG-F Genotype sp/sp;ko/ko
Available now
| Age in Weeks Post Engraftment | Quantity 1 - 999 |
|---|---|
| 12 to 26 | US$1,481.00 |
HSCCB-NOG-PREQC-F Genotype sp/sp;ko/ko
Cohorts are reserved upon order placement and will take 2-4 weeks to fulfill. An estimated lead time will be provided to you within 2-3 business days.
| Age in Weeks Post Engraftment | Quantity 1 - 999 |
|---|---|
| 3 to 9 | US$1,155.00 |
Pricing - EUR
Opportunist Free (OF) Health Standard
HSCCB-NOG Female
HSCCB-NOG-F Genotype sp/sp;ko/ko
Available now
| Age in Weeks Post Engraftment | Quantity 1 - 999 |
|---|---|
| 12 to 26 | 1.348,00 € |
HSCCB-NOG-PREQC-F Genotype sp/sp;ko/ko
Cohorts are reserved upon order placement and will take 2-4 weeks to fulfill. An estimated lead time will be provided to you within 2-3 business days.
| Age in Weeks Post Engraftment | Quantity 1 - 999 |
|---|---|
| 3 to 9 | 1.052,00 € |
Pricing - DKK
Opportunist Free (OF) Health Standard
HSCCB-NOG Female
HSCCB-NOG-F Genotype sp/sp;ko/ko
Available now
| Age in Weeks Post Engraftment | Quantity 1 - 999 |
|---|---|
| 12 to 26 | kr.10.024,00 |
HSCCB-NOG-PREQC-F Genotype sp/sp;ko/ko
Cohorts are reserved upon order placement and will take 2-4 weeks to fulfill. An estimated lead time will be provided to you within 2-3 business days.
| Age in Weeks Post Engraftment | Quantity 1 - 999 |
|---|---|
| 3 to 9 | kr.7.820,00 |
Pricing - USD Nonprofit
Opportunist Free (OF) Health Standard
HSCCB-NOG Female
HSCCB-NOG-F Genotype sp/sp;ko/ko
Available now
| Age in Weeks Post Engraftment | Quantity 1 - 999 |
|---|---|
| 12 to 26 | US$1,288.00 |
HSCCB-NOG-PREQC-F Genotype sp/sp;ko/ko
Cohorts are reserved upon order placement and will take 2-4 weeks to fulfill. An estimated lead time will be provided to you within 2-3 business days.
| Age in Weeks Post Engraftment | Quantity 1 - 999 |
|---|---|
| 3 to 9 | US$1,133.00 |
Pricing - EUR Nonprofit
Opportunist Free (OF) Health Standard
HSCCB-NOG Female
HSCCB-NOG-F Genotype sp/sp;ko/ko
Available now
| Age in Weeks Post Engraftment | Quantity 1 - 999 |
|---|---|
| 12 to 26 | 1.173,00 € |
HSCCB-NOG-PREQC-F Genotype sp/sp;ko/ko
Cohorts are reserved upon order placement and will take 2-4 weeks to fulfill. An estimated lead time will be provided to you within 2-3 business days.
| Age in Weeks Post Engraftment | Quantity 1 - 999 |
|---|---|
| 3 to 9 | 1.032,00 € |
Pricing - DKK Nonprofit
Opportunist Free (OF) Health Standard
HSCCB-NOG Female
HSCCB-NOG-F Genotype sp/sp;ko/ko
Available now
| Age in Weeks Post Engraftment | Quantity 1 - 999 |
|---|---|
| 12 to 26 | kr.8.722,00 |
HSCCB-NOG-PREQC-F Genotype sp/sp;ko/ko
Cohorts are reserved upon order placement and will take 2-4 weeks to fulfill. An estimated lead time will be provided to you within 2-3 business days.
| Age in Weeks Post Engraftment | Quantity 1 - 999 |
|---|---|
| 3 to 9 | kr.7.671,00 |
Select my Health Standard
Need help choosing the right Taconic Biosciences health standard for your research?
Use the Health Standard Selector to enter your exclusion list. The tool will tell you which health standards meet your requirements.
Get custom pricing guide
Schedule A Scientific Consultation
Connect directly with a member of our Scientific Solutions team who can help you select the most appropriate model and maximize your experimental success.
















.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)


.jpg)
.jpg)




.jpg)





.jpg)

.jpg)










