At the 2019 American Association for Cancer Research (AACR) conference, Dr. Kregel presented work describing a new humanized mouse model of metastatic castration resistant prostate cancer that appears to overcome many of the limitations in its predecessors. To help us understand how his research could help improve preclinical prostate cancer modeling, Dr. Kregel responded to questions about the work he presented at AACR 2019.
Q: Why do you believe the commonly-used metastatic prostate cancer mouse models are inadequate?
GEM models of metastatic prostate cancer have three main flaws:
- They rely on a mouse prostate that doesn't form sporadic tumors and differs anatomically and developmentally from the human prostate
- They lack human-disease heterogeneity and rarely metastasize
- Their disease progression is driven in a contrived manner unrelated to human disease and human disease drivers
Q: What improvements are needed for prostate cancer modeling and how did you plan research to address the needed improvements?
Our hypothesis was that humanizing tumor-immune interactions would improve modeling of metastatic prostate cancer, and perhaps improve modeling of hormonal and immune therapies. We generated a prostate cancer xenograft model in huNOG mice, which develop an intact human immune system from engrafted CD34+ stem cells, and we used this model to test our hypothesis.
Q: What was the approach used to evaluate your immune-humanized metastatic prostate cancer model?
The prostate cancer cells we used were engineered to express luciferase, which allows us to easily measure organ-specific metastatic growth at the end of the study. This design allowed us to observe how the presence or absence of a human immune system impacts growth at the primary-injection site, metastasis and metastatic outgrowth, and enzalutamide response.
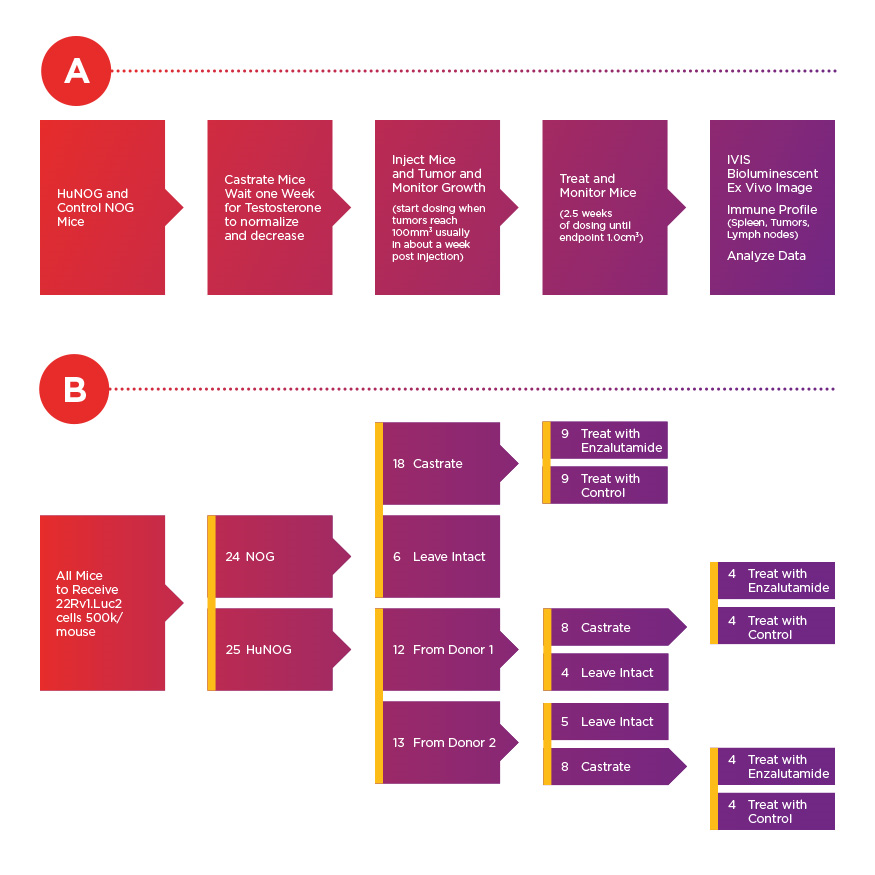
Experimental timeline (A) and conditions (B) for assessment of 22Rv1.luc2 xenografts cells. According to Dr. Steve Kregel, 22Rv1 cells are among the most aggressive prostate cancer cell lines in vivo that still have androgen receptor expression.
Q: How would you summarize your key observations?
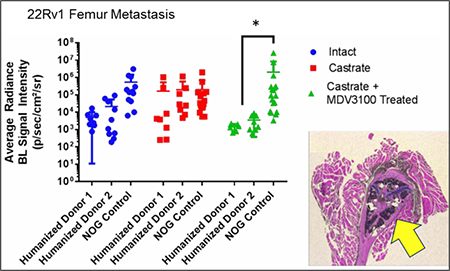
University of Michigan researchers observed a significant increase in the
metastatic burden of NOG control mice castrated and treated with
enzalutamide, and a significant decrease in metastatic outgrowth in
enzalutamide-treated huNOG mice. Cancer cells were seen in the bone
marrow and matrix of the epiphyseal head of a mouse femur
(yellow arrow indicates 22Rv1 tumor mass). Femoral metastases were
confirmed by histology (H&E stain) and a pathologist's assessment.
We observed reduced metastatic growth following enzalutamide treatments in two different cohorts of huNOG mice, each produced with human stem cells from a different human donor. In a sense, each cohort acts as an avatar for the donor's immune system and for modeling how it might interact with prostate cancer. Our results align with recently published clinical data that suggests enzalutamide prevents metastatic growth in patients, and further suggest that anti-metastatic effects from enzalutamide could be mediated by the immune system.
Q: Were there any other notable observations from your work you can share?
Another interesting observation was increased T-cell infiltration in the tumors of the huNOG mice treated with enzalutamide when compared to hormonally intact or castrated mice. The T-cells infiltrating the tumors also expressed more interferon-γ, which is a read-out of their activation, and suggests that enzalutamide has the potential to help activate the immune system in patients. More work is needed to assess whether this is directly mediating the response seen with the decrease in metastases, or if the enzalutamide is acting directly on the immune cells, or if the mechanism is more complicated through tumor- and immune-cell cross talk.
Q: What are you next steps for this system?
There are numerous other approaches I'd like to apply and study with these models, including the huNOG-EXL model and similar hosts expressing human cytokines for better human myeloid cell engraftment. For example, we currently have focused a lot on the lymphoid activation, but if we want to investigate novel therapies, such as STING agonists, or how radiation and chemotherapy impact immune activation in prostate cancer, huNOG-EXL or other models that are better at engrafting human myeloid cells might be necessary.
Q: How do you see this model impacting preclinical prostate cancer research?
These models should be adaptable to nearly any human tumor and can model heterogeneity and response to the current standard of care AR-targeted therapies. GEM models clearly cannot do all of that. For researchers developing therapeutics for metastatic castration resistant prostate cancer, our system and approach is immediately worth considering adopting. Future work might lead to this type of approach becoming the gold standard for certain pre-clinical in vivo modeling.
















.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)


.jpg)
.jpg)




.jpg)




.jpg)

.jpg)




