The glial cells
play pivotal roles in the central nervous system (CNS) by supporting its development, maintaining homeostasis, producing myelin, and providing support and protection for neurons
1. These cells are increasingly under scientific scrutiny in the pathogenesis of
neurodegenerative disorders (ND).
The activated microglia, a subset of glial cells, have been demonstrated to be the
main determinant of neuroinflammation that contributes to neurodegeneration
2.
Increasing evidence supports the interactions of various microorganisms with the human immune system in contributing (directly and indirectly) to the onset and severity of ND
3.
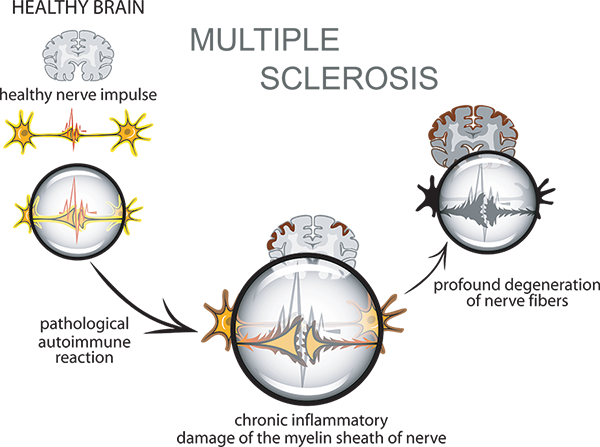
Examining MS Pathogenesis in Mouse Models
Multiple sclerosis, the most frequent chronic inflammatory disease of the CNS, is
a demyelinating disease of
inexorable damage to the insulating protection of neurons within the central and peripheral nervous system (CNS and PNS)
4,5. One important driver of MS pathogenesis and potential therapeutic target are the
B-cells and their effector subsets,
plasmablasts (PBs) and plasma cells (PCs).
In a recent attempt to determine the source and role of these cells in the CNS of MS patients, Gommerman and co-workers
utilised multiple approaches, including the
adoptive transfer experimental autoimmune encephalomyelitis (EAE) model, to elucidate their source and function, as well as clarify their associations to the microbiota in the CNS during neuroinflammation
6.
They uncovered that some PCs in the CNS of EAE mice originate in the gut and produce immunoglobulin A (IgA). The presence of IgA+ PBs and PCs in the CNS was unexpected, given that IgA is principally produced and secreted at mucosal surfaces. In addition, the authors found that these gut-derived IgA+ B cells were able to attenuate inflammation in the CNS. Therefore, even though IgA-producing PC are well-known to produce copious anti-commensal antibodies in the gut during homeostasis, these new results should evoke an emendation of these cells during autoimmune and neuroinflammatory diseases.
Does IL-10 Expression Ameliorate MS Symptoms?
Importantly, MS patients exhibited an abatement of fecal bacteria-bound IgA during relapses, possibly due to egress of IgA-producing cells from the gut. Gommerman's group discovered that the expression of IL-10 by PCs was integral for the amelioration of EAE, which were findings consistent with previous work by Matsumoto et al.
7.
However,
in another study, Zhou et al. reported BAFF-Tg mice exhibited exacerbated EAE that correlated with increased Th17 cells in the draining lymph nodes
8. These divergent results and reports suggest that
distinct vivarium-associated microbiota, which is known to affect IgA+ antibody-secreting cells in the CNS, require further studies.
 View the Taconic Biosciences' Webinar:
View the Taconic Biosciences' Webinar:
An Unexpected Role in Neuroinflammation
Gommerman's group clearly demonstrated that gut-mobilized IgA+ PB and PC play an unexpected but important role in dampening neuroinflammation. Therefore, improved appreciation of the associations between ND and microorganisms, within the context of the immune system would enable researchers in the development of novel therapies to better diagnose, prevent, and manage neurodegenerative and neuroinflammatory disorders.
 Download the Taconic Biosciences White Paper:
Download the Taconic Biosciences White Paper:
References:
1. Jessen KR, Mirsky R (August 1980). "Glial cells in the enteric nervous system contain glial fibrillary acidic protein". Nature. 286 (5774): 736-7. doi:10.1038/286736a0. PMID 6997753.
2. Aging Dis. 2018 Dec 4;9(6):1134-1152. doi: 10.14336/AD.2018.0201. eCollection 2018 Dec. Molecular Bases of Alzheimer's Disease and Neurodegeneration: The Role of Neuroglia. Luca A1, Calandra C1, Luca M2. https://doi.org/10.14336/AD.2018.0201.
3. Front Cell Neurosci. 2018 Dec 4;12:466. doi: 10.3389/fncel.2018.00466. eCollection 2018. Microorganisms' Footprint in Neurodegenerative Diseases. Dehhaghi M1,2, Kazemi Shariat Panahi H1,2, Guillemin GJ1. https://doi.org/10.3389/fncel.2018.00466.
5. Nat Rev Neurol. 2014 Apr;10(4):225-38. doi: 10.1038/nrneurol.2014.37. Epub 2014 Mar 18. Mechanisms of neurodegeneration and axonal dysfunction in multiple sclerosis. Friese MA1, Schattling B1, Fugger L2. https://doi.org/10.1038/nrneurol.2014.37
6. Cell. 2018 Dec 21. pii: S0092-8674(18)31560-5. doi: 10.1016/j.cell.2018.11.035. [Epub ahead of print]. Recirculating Intestinal IgA-Producing Cells Regulate Neuroinflammation via IL-10. Rojas OL1, Pröbstel AK2, Porfilio EA1, Wang AA1, Charabati M3, Sun T1, Lee DSW1, Galicia G1, Ramaglia V1, Ward LA1, Leung LYT1, Najafi G1, Khaleghi K1, Garcillán B4, Li A5, Besla R6, Naouar I1, Cao EY1, Chiaranunt P1, Burrows K1, Robinson HG7, Allanach JR7, Yam J1, Luck H5, Campbell DJ8, Allman D9, Brooks DG10, Tomura M11, Baumann R2, Zamvil SS12, Bar-Or A13, Horwitz MS14, Winer DA6, Mortha A1, Mackay F4, Prat A3, Osborne LC7, Robbins C15, Baranzini SE16, Gommerman JL17. https://doi.org/10.1016/j.cell.2018.11.035.
7. PLoS One. 2011;6(8):e23629. doi: 10.1371/journal.pone.0023629. Epub 2011 Aug 29. BAFF promotes Th17 cells and aggravates experimental autoimmune encephalomyelitis. Zhou X1, Xia Z, Lan Q, Wang J, Su W, Han YP, Fan H, Liu Z, Stohl W, Zheng SG. https://doi.org/10.1371/journal.pone.0023629
8. Immunity. 2014 Dec 18;41(6):1040-51. doi: 10.1016/j.immuni.2014.10.016. Epub 2014 Nov 4. Interleukin-10-producing plasmablasts exert regulatory function in autoimmune inflammation. Matsumoto M1, Baba A2, Yokota T3, Nishikawa H4, Ohkawa Y5, Kayama H6, Kallies A7, Nutt SL7, Sakaguchi S4, Takeda K6, Kurosaki T8, Baba Y9. https://doi.org/10.1016/j.immuni.2014.10.016.







.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)
.jpg)


.jpg)


.jpg)




.jpg)

.jpg)
.jpg)





