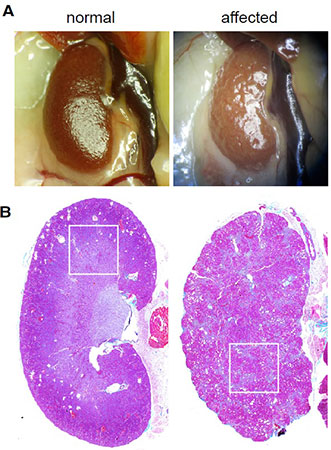
The kidneys of immunodeficient mice infected with
MKPV appear pale and shrunken on gross necropsy.
Histology reveals significant fibrosis.
Source: Roediger et al. Cell 20181.
Impact on Immunodeficient Animals
Mice infected with MKPV display kidney anatomical and functional abnormalities including tubular degeneration, necrosis and fibrosis as well as elevated serum creatinine and blood urea nitrogen. Affected mice may also lose weight and become anemic1.Immunocompetent mice may be infected with MKPV, but show minimal clinical illness, with some animals displaying moderate nephropathy. Affected nude mice (which lack T cells) are reported to show mild nephropathy. In more severely immunodeficient mice such as Rag1 knockouts, scids or NSG mice, MKPV causes more significant nephropathy and may cause death. Affected animals may experience kidney dysfunction for 4-5 months prior to death1.
How is MKPV Transmitted?
The virus is transmitted via fecal-oral or urinary-oral routes and is highly infectious. Roediger et al. state that "infection is likely established by the time of weaning (3-4 weeks) and that MKPV is highly penetrant in immunodeficient mice1."MKPV Prevalence in Animal Research Colonies
Immediately following the discovery of this new pathogen Taconic Biosciences' scientists developed a testing strategy for commercial mouse colonies. "We initiated screening of all commercial mouse colonies in January of 2019, including those located at our global production partner facilities in Asia," said Dr. Meggan Keith, Director of Diagnostics and Genetics at Taconic. "We were pleased to find that all commercial colonies are negative for MKPV. Because our strict bioexclusion practices are designed to prevent viral entry into barriers, we expected negative findings," said Dr. Keith.
What MKPV Means for Veterinarians and Colony Managers
Because of the significant clinical illness caused by MKPV, many facilities may wish to exclude this pathogen, particularly from their immunodeficient mice as MKPV can cause morbidity and mortality in such mice. Roediger et al. note that "the long-term persistence of MKPV within several immunodeficient lines indicates this virus, once established, is not readily removed from host facilities without rederivation. This strongly suggests that the detection in a single immunodeficient mouse will imply likely MKPV infection across the entire colony1."There is currently no data available regarding the feasibility of efforts to eliminate MKPV from contaminated facilities. Assessing facility status using diagnostic tools is the first step. At least one animal health testing laboratory currently offers a PCR-based assay to detect MKPV in feces, urine, serum or kidney tissue. The availability of MKPV-free mice with which to restock a facility is critical to elimination of the agent. Taconic now excludes MKPV from all Taconic health standards, meaning that facilities may be immediately restarted with virus-free mice, including sentinels and immunodeficient mice. Rederivation of specialty strains not available from a commercial vendor may be required. While rederivation with virus-free recipient dams is considered the gold standard in eliminating infectious agents from colonies, reports of murine parvovirus adhering to embryos even after wash steps indicate care should be taken to adequately assess viral status of rederived mice prior to restarting a colony4. Consult your facility veterinarian for additional discussion, including development of an effective testing strategy with regards to use of sentinels versus line animals and sample type.
















.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)


.jpg)
.jpg)




.jpg)





.jpg)

.jpg)





