Checkpoint inhibitors work indirectly. CAR T cells work directly, transforming a patient's own T cells into a therapy by improving their ability to bind and destroy cancer cells. This approach is referred to as a type of adoptive cell transfer therapy, because the patient's own cells are genetically modified and then transferred back into the patient.
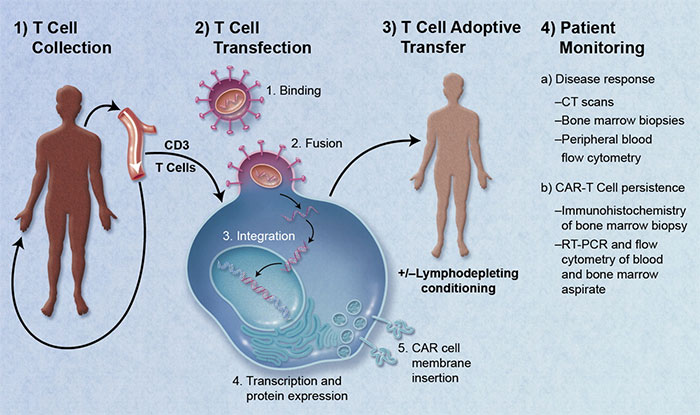
Credit: Blood 2011 118:4761-4762
How Does CAR T Cell Therapy Work?
Chimeric Antigen Receptor (CAR) modified T cells, or CAR T cell therapy, uses a patient's own T cells to fight cancer. The patient's own T cells are re-engineered to express antigen receptors that improve binding of T cells to cancer cells. It is a way of boosting the cancer cell killing attributes of T cells.To accomplish this feat, white blood cells are removed from the patient and T cells are isolated. The T cells are then genetically modified to express chimeric antigen receptors on their surface. The modified T cells are expanded in bioreactors, then injected back into the same patient. Once back in the patient, the modified T cells expand and preferentially bind with the cancer cells. The T cells then do their job to destroy the cancer cells.
In August of 2017, Novartis received FDA approval for the first CAR T cell therapy, Kymriah™, which targets a form of leukemia. In three months, 83% of patients were in complete remission. These were patients that did not respond to other more traditional cancer therapies. Not long after the approval of Kymriah, Gilead Science's Yescarta® received approval from the FDA.
Unlike standard bulk drug manufacturing, CAR T cell therapy is made for each individual patient. A single injection of Kymriah can cost close to $500,0001.
Is CAR T Cell Therapy Safe?
CAR T cell therapy makes perfect sense: stimulate the body's ability to recognize and bind cancer cells. Yet, this improvement can also cause the patient's immune system to run amuck in a so-called cytokine storm — which can be fatal. These side effects have been implicated in deaths of some patients treated with CAR T. With a high cost and high risk, CAR T cell therapy is often a treatment of last resort.Fortunately, scientists have begun to develop risk-mitigating technologies. Scientists are developing control systems that can turn the cells "off" if a cytokine storm is detected.
T cells multiply when they recognize antigens they view as foreign. With engineered T cells, this reaction can be even more powerful. While this might be beneficial — the more T cells that bind cancer cells, the better the chance of killing them — when T cells bind other cells, they release cytokines. Cytokines promote inflammation and attract more immune cells to the area. When this occurs, it can result in a fatal condition called a cytokine flare-up.
Improving Safety in CAR T cell Therapy
Biotech companies are developing ways to either control the T cell reaction or, if needed, turn it off. One company is Bellicum Pharmaceuticals based in Houston, Texas. Their "product candidates are differentiated by the inclusion of powerful molecular switches designed to eliminate, reduce or activate therapeutic cells, and thereby potentially provide greater efficacy and safety compared to the current generation of cell therapies." They have included molecular switches which can be controlled inside the patient by administering rimiducid, a small molecule.Depending on the type of engineered molecular switch, administration of rimiducid can lead to T cell death, beneficial for patients that develop a cytokine storm, or rimiducid could cause proliferation of T cells with an activation switch.
Other technologies in development are focused on making CAR T cell therapy even more defined. Cell Design Labs (now owned by Gilead) engineered a receptor called synNotch into T cells. When the receptor binds a surface protein on the cancer cell, the receptor activates, prompting CAR production on the surface of the T cells.
Two recent findings published in Nature Medicine also highlight the need for including a kill-switch into CAR-T platforms in order to engineer a level of safety into cell therapies. Groups at Novartis2 and Karolinska Institute3 independently found that CRISPR/Cas9-edited cells are most efficiently modified when the proto oncogene p53 malfunctions.
This is important for three reasons:
- First, since p53 is involved in sensing DNA repair, it can instruct a "fix or kill" pathway where cells experiencing DNA breaks either repair the break or undergo programmed cell death by apoptosis.
- Second, since CRISPR/Cas9 works by tactically instructing DNA breaks, the p53 pathway plays a central role in the fate of a gene-modified cell.
- Third, as CRISPR/Cas9 modifications are necessary for engineering some types of CAR-T cells, and p53 malfunctions lead to more efficient gene modifications, the newly-modified cells bear an increased propensity to propagate a p53 malfunction.
Preclinical Models for CAR T cell research
Super-immunodeficient rodent models, like the CIEA NOG mouse®, support the engraftment of human tumors and functioning human immune cells through several distinct approaches. These tumor-engrafted humanized immune system models are now used in preclinical evaluations of T cell-dependent cancer therapies, including checkpoint inhibitors, CAR T cells, and T cell-engaging multi-specific antibodies.While T cells remain central to many ongoing immuno-oncology efforts, scientists are adapting T cell methodologies toward other immune cells types, such as NK cells. Examples include NK cell-engaging multi-specific antibodies and NK cells genetically engineered to have enhanced tumor-killing properties, much like CAR T cells.
For T cell immunotherapies, HLA transgenic mice are also used to identify sequences for high-affinity T cell receptors, enabling development of new CAR T cell therapies.
These recent approvals have advanced the treatment of cancer in remarkable ways. Enlisting the patient's immune system to help battle cancer is just beginning and the future holds great opportunities.
















.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)


.jpg)
.jpg)




.jpg)





.jpg)

.jpg)




