Pre-clinical studies in the APPSWE/Tg2576 mouse model of AD provided the mechanistic clues, dosing insights, and efficacy data that informed the design of Biogen's successful aducanumab Phase I trial. Now, with FDA Fast Track designation, aducanumab is progressing through large Phase III trials.
By analyzing and understanding Biogen's preclinical approach and data, researchers may be able to improve the design of their own Alzheimer's disease model studies.
Preclinical Investigation in a Rodent Alzheimer's Disease Model
Reported alongside favorable clinical data in their 2016 article, Biogen investigators assessed chaducanumab, a mouse-optimized version of aducanumab, for its ability to reduce Aβ-burden in Tg2576 mice.At nine months of age, male and female Tg2576 mice received a weekly intraperitoneal (IP) dose with PBS, 0.3, 1, 3, 10, or 30 mg/kg of chaducanumab. Following six months of chronic treatments in the mice, researchers reported:
- chaducanumab reduced amyloid burden in Tg2576 mice in a dose-dependent manner.
- chaducanumab penetrated the brain to a sufficient extent to allow accumulation on Aβ plaques.
- The observed dose-response in Tg2576 mice was consistent with the clinical doses that led to reductions in Aβ.
- chaducanumab cleared plaques of all sizes, suggesting that aducanumab aided in clearing pre-existing plaques and prevented new plaque formation.
- The clearance of Aβ deposits was associated with enhanced recruitment of microglia.
Evidence for Efficacy Without Reducing Amyloid
In a later-published collaboration, Harvard and Biogen scientists evaluated the effects of chaducanumab on calcium homeostasis and plaque clearance in aged Tg2576 mice.At eighteen months of age, mice began weekly IP injections of chaducanumab or control antibody (10mg/kg). Following six months of chronic chaducanumab treatments in aged (eighteen-month-old) Tg2576 mice, researchers reported:
- Amyloid burden in aged Tg2576 mice was not affected by chaducanumab treatments.
- The presence of calcium overload was verified in neurites of aged Tg2576 mice.
- Despite not affecting pre-existing plaques in aged Tg2576 mice, chaducanumab normalized neurite calcium overload early throughout the course of treatment.
- chaducanumab reversed NMDA receptor downregulation in the brains of aged Tg2576 mice.
- Calcium overload may be a useful outcome measure to monitor treatment efficacy in AD models.
Comparing Data from Young and Aged Tg2576 Mice
chaducanumab's ability to reduce amyloid burden in Tg2576 mice was dependent on the age at which mice began treatment (Figure 1). Following six months of chronic chaducanumab treatments, amyloid burden was significantly reduced in mice that began their treatment at nine months of age, but not in equivalently-dosed mice that began treatments at eighteen months of age.These results are consistent with the notion that immunotherapy is less effective in advanced AD stages with substantial plaque deposits. However the beneficial effect of chaducanumab on neurite calcium levels in aged Tg2576 mice was observed despite no reductions in brain amyloid burden. This result suggests amyloid burden may be an inadequate efficacy metric.
One explanation for the discrepancy between plaque and calcium reductions is that the beneficial effects from chaducanumab are mediated predominantly through its specificity for Aβ oligomers, as opposed to Aβ plaques. Indeed Aβ oligomers are now commonly thought to be the Aβ species responsible for AD-associated neurodegeneration. Thus, clearance of Aβ plaques may be a secondary effect of aducanumab treatment, with primary effects mediated through reduction of Aβ oligomers.
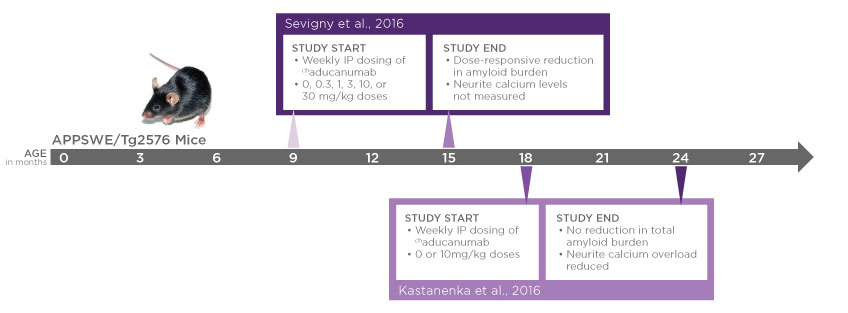
Figure 1
Preclinical Insights from Rodent Alzheimer's Disease Models
Biogen's preclinical studies reveal potentially important AD mechanisms and provide useful clues that can guide study design. Important insights that can impact preclinical studies include:- Immunotherapies can cross the blood brain barrier (BBB) in Tg2576 mice to influence amyloid burden and neurite functional measures.
- In the window between nine and fifteen months of age, it's possible for an immunotherapy to reduce amyloid burden in Tg2576 mice.
- With age and increased amyloid deposition, it becomes more challenging to reduce amyloid burden in Tg2576 mice.
- Positive functional effects can be observed without reducing amyloid burden.
- Future work will determine whether neurite calcium levels can be used to monitor treatment efficacy in young Tg2576 mice and in other AD models.
- If reducing amyloid burden is a desired study outcome, researchers should focus on younger Tg2576 mice, and should also consider including additional efficacy metrics (e.g. neurite calcium levels) in their study.
- For testing therapies directed at later-stage AD, Tg2576 mice around eighteen months of age could be a suitable model wherein amyloid burden would not necessarily be affected by treatments.
- For testing immunotherapies, the established permeability of the BBB to monoclonal antibodies in Tg2576 mice is a desirable trait that enhances the utility of the model.
















.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)


.jpg)
.jpg)




.jpg)




.jpg)

.jpg)



