New Mouse Model for Parkinson's Disease Research
No single Parkinson's Disease (PD) model fully reproduces the complexity of the disease in humans. Developing an assortment of PD models has been essential for furthering our understanding of, and the development of potential treatments for, the disease.Adding to the arsenal of PD rodent models, in conjunction with The Michael J. Fox Foundation (MJFF) for Parkinson's Research, a mouse model carrying targeted replacement human LRRK2 G2019S alleles is now available to non-profit and industry researchers.
In recent work from a team of Mount Sinai investigators, the new Parkinson's disease model helped identify an important role for the G2019S mutation in altering neuronal activity and structures. This research identifies early postnatal alterations associated with the LRRK2 G2019S mutation that may predispose to later neuronal circuit dysfunction.
New Model Tames LRRK2 Kinase Over-expression
Mutations in the leucine-rich repeat kinase 2 (LRRK2) gene are a known cause for autosomal dominant inherited PD. Among the known LRRK2 mutations, the G2019S point mutation is most frequent and results in increased LRRK2 kinase activity.Several PD transgenic rodent models carrying the G2019S mutation have been created, including the G2019S rat and Lrrk2 deficient mice, which lack the kinase. Transgenic expression of a protein is associated with relatively high protein-expression levels, altered expression patterns, and distribution throughout tissues. Thus, the regulation of the transgene (i.e. overexpression and tissue specificity) remains a limitation in transgenic G2019S rodent models.
A team of Mount Sinai scientists, led by Dr. Deanna Benson and supported by the MJFF, described a novel and unique tool for PD research: a mouse model carrying the human LRRK2 G2019S mutant kinase under endogenous regulation.
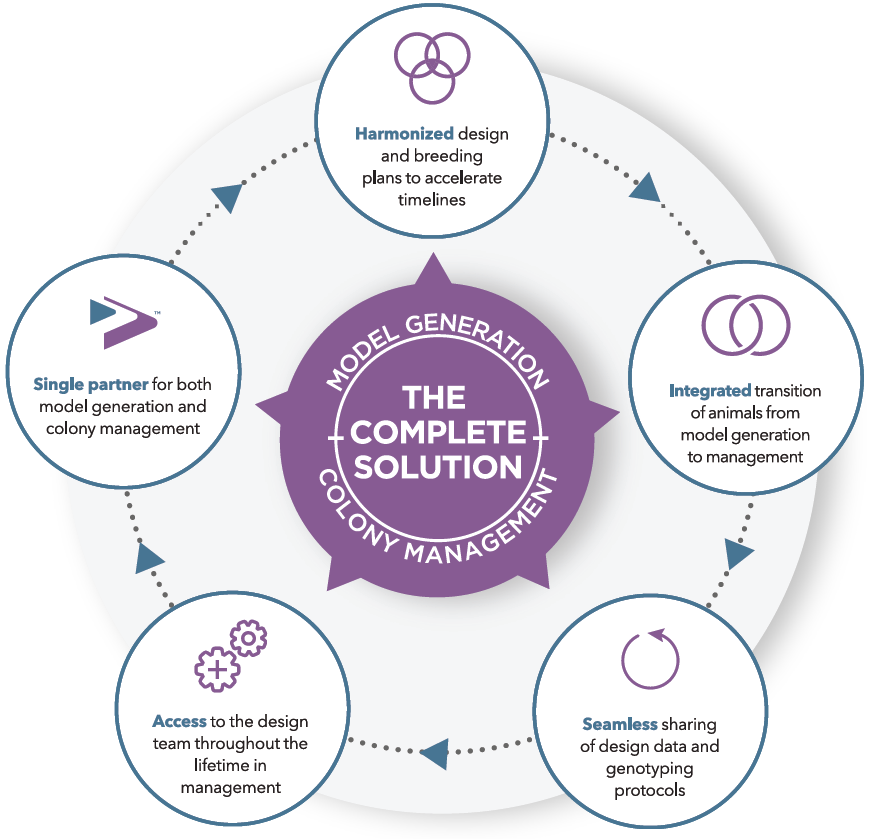
This targeted replacement model overcomes the regulation limitations associated with transgenic models, adding an important new tool for PD research.
Insights from LRRK2 G2019S Targeted Replacement Mice
In their evaluation of the LRRK2 G2019S mouse model, Dr. Benson's team described these insights from the PD model:- Stained coronal sections of the brain revealed relatively normal cytoarchitecure in LRRK2 G2019S mice.
- The expression levels of the mutant human protein in G2019S mice was similar to levels detected in wildtype mice.
- There were no differences observed in the levels of tyrosine hydroxylase, a marker for dopaminergic neurons.
- The G2019S mice displayed increased spontaneous excitatory postsynaptic current (sEPSC) frequency in dorsal striatal spiny projection neurons (SPNs).
- The abnormal activity in SPNs could be blocked by a LRRK2 kinase inhibitor.
- Physically isolating the dorsal striatum from cortex normalized sEPSC frequency in SPNs.










.jpg)

.jpg)
.jpg)
.jpg)
.jpg)



.jpg)


.jpg)
.jpg)


.jpg)



.jpg)

.jpg)



