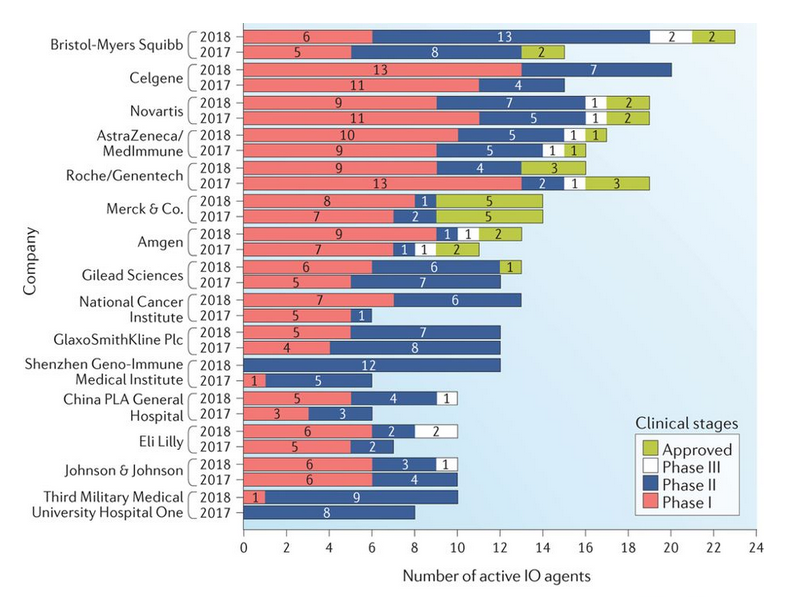
Figure 1: Companies actively pursuing immunotherapies.
Source: Trends in the Global Immuno-oncology Landscape (2018)
Immunotherapy research continues to explode. There are close to thirty approved drugs that stimulate the immune system to kill cancerous cells1. As of September 2018, there were over 3,300 active immuno-oncology agents in development with over 600 organizations developing clinical drugs2.
James P. Allison and Tasuku Honjo received the 2018 Nobel Prize for Medicine for their contributions in the field of immuno-oncology research.
How Big is the Cancer Immunotherapy Market?
Transparency Market Research estimates the cancer immunotherapy market size will reach $124 billion by 2024, with a CAGR of nearly 15% from 2016. Meticulous Research puts the number closer to $152 billion by 2024. Both reports cite North America as having the largest share, followed by Europe and Asia-Pacific. According to the Meticulous Report:Passive and Active Immunotherapies
There are two categories of immunotherapy:- passive
- active immunotherapy
The specific cancer type, the plan of attack, and limitations of today's technology drive the choice of which immunotherapy to employ.
For a concise overview of immunotherapy, including background information on the immune system see About Immuno-Oncology. For a more detailed look at immunotherapy, including areas of focus to make further advancements and create positive outcomes for a wider patient population, see Allard (2018)3. One area of focus is the identification of more biomarkers. The most well-known biomarkers at present are PD-L1 and TIL and can indicate response to immunotherapy. Identification of additional biomarkers will help clinicians decide on the best treatment options.

Figure 2: Number of active IO agents by therapy type. Source: Trends in the Global Immuno-oncology Landscape (2018)
Which Immunotherapy is Appropriate?
The Cancer Research Institute provides details of how immunotherapy treatment is impacting specific cancer types. Some approved drugs treat one cancer type while others, such as pembrolizumab (Keytruda®, Merck), treat multiple cancer indications. The page also includes ongoing clinical trials by treatment type, such as Targeted Antibodies, Cancer Vaccines and Adoptive Cell Therapy.Animal Models in Immunotherapy Research
Super-immune deficient mice, such as the CIEA NOG mouse® engrafted with a human immune system (HIS), make effective preclinical immunotherapy research possible. The ability to recapitulate the human immune system in mice allows investigators to evaluate the efficacy and safety of new drug entities, especially the biologics used in immunotherapy.The ability to implant and grow cancer cells in mice also supports immunotherapy research. Through the use of xenografts, investigators can implant patient-derived cancer tissue into mice and then apply various treatment regimens to see which one is most effective in reducing or eliminating the cancerous tissue. This benefits patients by providing them the best treatment options rather than a "try and see" approach.
















.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)


.jpg)
.jpg)




.jpg)





.jpg)

.jpg)