This has involved a paradigm shift in how scientists approach cancer research. For decades, the field has been dominated by studies focusing on the tumor itself to identify specific targets that would directly slow cancer growth. In immuno-oncology, the tumor per se is less important than how it interacts with the immune system, both locally (i.e. the tumor microenvironment) and systemically (i.e. cell-mediated and humoral immune responses). This has required a shift from traditional xenograft studies in immunodeficient hosts, to developing syngeneic tumor models in which to engage the host immune responses.
While still in its relative infancy, immuno-oncology research identified numerous promising therapeutic strategies, including checkpoint blockade inhibitors targeting CTLA-4 or PD-1. Normally, CD8+ T cells, a powerful effector cell type of the adaptive immune system, are prevented from reacting to tumors. Checkpoint blockade inhibitors act by allowing the patient's own T cells to mount a response to a tumor and attack it.
While results from checkpoint inhibitor trials are promising, it should be noted that this strategy alone may have therapeutic limitations. Advanced tumors often possess numerous immunosuppressive mechanisms to evade attack from the immune system. Tumor immune evasion remains a major challenge for immuno-oncology, and one focus of combination immunotherapy research is circumventing these multiple immunosuppressive pathways.
Preclinical Success in Combination Immunotherapy Research
A new preclinical study by Kelly Moynihan and colleagues at the Massachusetts Institute of Technology has identified an effective combination immunotherapy strategy that holds promise for treating large advanced tumors. Their treatment included a four-pronged approach:- A novel, lymph node-targeted tumor vaccine, in combination with:
- A tumor-specific antibody;
- A checkpoint blockade inhibitor (anti-PD-1) and;
- A cytokine to boost immune responses (long acting IL-2).
- B16F10 melanoma cells injected into syngeneic C57BL/6NTac mice
- DD-Her2/neu breast cancer cells injected into syngeneic BALB/c mice
- TC-1 cells expressing human papilloma virus (HPV) oncogenes injected into syngeneic C57BL/6NTac mice
Interestingly not only were the mice cured of initial tumors, but they were also protected against subsequent attempts to reintroduce the same tumors. This "immunological memory" suggests that similar treatment strategies in patients might not only treat primary tumors, but may also prevent recurrence.
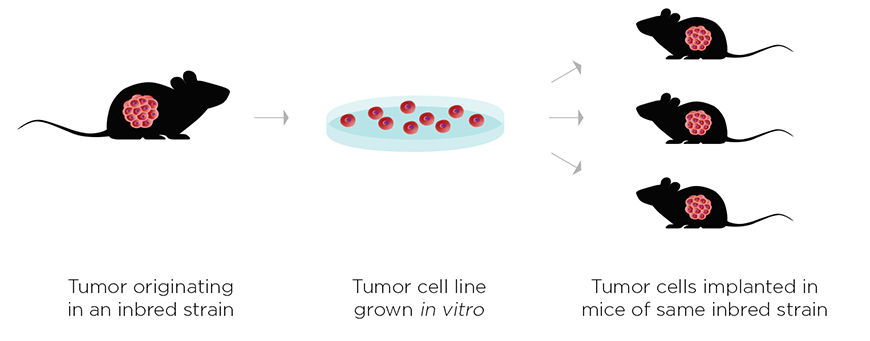
A key to the success of this study was the authors' use of syngeneic models, which possess a complete and normal immune system. Their combination strategy worked by acting on a diverse repertoire of adaptive and innate immune cells. In addition to CD8+ T cells, the authors found that innate effector cells (neutrophils, macrophages, natural killer cells, and cross-presenting dendritic cells) were also required to drive an effective and durable anti-tumor response.
The power of using syngeneic models for combination immunotherapy research is the ability to engage a wide repertoire of different immune cell types. According to lead author Kelly Moynihan, "Syngeneic models are critical for immuno-oncology preclinical research because you need both a fully intact immune system and MHC haplotype-matched tumors to get the most meaningful and informative results. It's becoming very apparent that a significant and complex relationship exists between immune cells and tumor cells, and xenograft models ignore this very important immunological contribution."
In working to translate these important findings into effective therapies for patients, it will be important to study these responses in the context of human tumors and the human immune system. The Taconic huNOG portfolio offers the ability to study how the human immune system reacts with either cell line- or patient-derived tumors, in order to determine how next-generation combination therapies could be translated to clinical use.
















.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)


.jpg)
.jpg)




.jpg)





.jpg)

.jpg)




