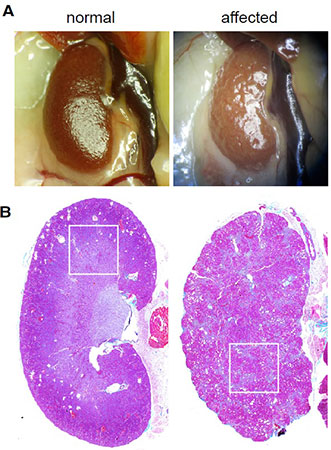
The kidneys of immunodeficient mice infected with MKPV appear pale and shrunken on gross necropsy. Histology reveals significant fibrosis. Source: Roediger et al. Cell 20181
AALAS discussions: testing challenges and exclusion decisions
MKPV was a hot topic at the 70th Annual National AALAS Meeting in October 2019, with a platform session entitled "Murine Chapparvovirus aka Mouse Kidney Parvovirus: A Novel Virus Identified Using Metagenomics" devoted to it. Scheduled for 2 hours and 15 minutes, the session ran over by nearly 30 minutes due to extended audience dialogue. Presenters included Neil Lipman from Memorial Sloan Kettering, Kenneth Henderson from Charles River and Robert Livingston from IDEXX BioAnalytics among others. Multiple speakers discussed efforts towards developing improved detection methods. One of the key take-home messages from the presentations was that although the timeline of viral shedding is consistent with transmission to sentinels during a normal quarantine period, exposed mice develop antibodies relatively late. Thus although PCR can reliably detect MKPV presence, quarantine programs which rely solely on serology may miss MKPV infection.In response to an audience question about the status of vendor colonies for MKPV, Ken Henderson of Charles River represented the vendor perspective. He noted that Taconic Biosciences had announced testing and exclusion of MKPV. Regarding Charles River colonies, he said, "Anything raised in isolators, all of our immunodeficient and standard elite strains, founder colonies — those are all negative. We do have a handful of barriers that are positive." He was not aware of public responses from other vendors at that time.
The question of whether to exclude MKPV from animal facilities was addressed. Of the speakers, Robert Livingston of IDEXX strongly recommended exclusion of MKPV from immunodeficient mice based on the clinical impact, but was more circumspect when it came to immunocompetent mice. "In immune deficient mice, principally lacking adaptive immune system: it's contagious, it's a persistent infection causing chronic renal disease and morbidity, weight loss and death as the animals age. Considering those criteria, my thought is we probably do want to exclude that pathogen from a mouse colony. Seems like a no brainer to me ... What about immune competent mice? I think a lot of the same pieces hold true...It's a prolonged shedding, prolonged infection, may not be persistent. It certainly affects the kidney in immunocompetent mice...the kidney is involved in host homeostasis. For drugs, PK studies — there are drugs that are actively excreted through the renal tubular epithelium. While we're not seeing clinical signs, for those reasons, if I was an investigator looking at PK studies or renal physiology certainly I would be concerned." He continued, "From strictly a disease standpoint, I think this agent will make it onto our excluded pathogen list."
Henderson explained that exclusion decisions at Charles River would be based on customer demand. "We're actually providing information to our clients, people who really want to know. The strains in those rooms are available at other locations, and people can get them from different sites if they want to," he said. "We're still engaging with the community, how they're going to respond to this; if it's a virus people are going to be eliminating from all their facilities or some parts...whether from a production standpoint that's going to be necessary or not. We do have long term plans to evaluate those rooms and, if necessary, replace the colonies."
Jeffrey Lohmiller of Taconic added another vendor view, commenting that while most customers would like mice free of all identified agents, there is a cost associated with testing and exclusion, and that cost must be balanced with the need for affordable laboratory mice. No comment was made by representatives from other animal vendors. The session recording is available to view for those interested in the full discussion (note a fee is required and the content is only available to AALAS members).
New research
A January 2020 publication explored MKPV propagation in mouse hosts and compared the mouse virus to parvoviruses adapted to other mammal hosts. "Murine and related chapparvoviruses are nephro-tropic and produce novel accessory proteins in infected kidneys" was published in PLOS Pathogens. Lee et al. demonstrated that MKPV was present in various wild mouse populations in the US and China, with infection of laboratory mouse colonies via wild mice likely occurring in the US repeatedly over time. In contrast, the source of MKPV infection in Australian laboratory colonies appears to be related to a single importation event from the US. Analysis of MKPV RNA showed that the virus preferentially replicates in the kidney compared to other tissues, and that related viruses may also be kidney-tropic in their hosts2.Changes to facility exclusion lists?
The challenge with a newly discovered agent which has already infiltrated animal facilities is the time, expense and effort required to remove and exclude that agent. At least in the short term, many facilities are likely to take a practical approach. For research involving immunocompromised mice for which MKPV is a clinical concern or pharmacokinetics and kidney biology studies for which MKPV may confound results, researchers will likely exclude it. Outside of those categories, veterinarian and facility managers may continue to tolerate it — at least until otherwise-scheduled facility rotations and restarts provide an opportunity to remove it. Availability of mice free of the agent from commercial vendors is a key requirement to support clean up of user facilities.In May 2020, the Jackson Laboratory announced that its mice were free of MKPV and that it had added MKPV testing to JAX health reports, becoming the second vendor (after Taconic) to report on and exclude this agent.
Some institutions have performed surveillance, but not taken action to remove MKPV from their facilities. University of California Davis assessed the scope of the problem and worked collaboratively with its investigators. It first assessed prevalence of MKPV in its facilities via additional screening for one year. MKPV was found in several rodent rooms in two different buildings. UC Davis did not add the agent to its exclusion list. According to Dr. Laurie Brignolo, Executive Director of the UC Davis Research and Teaching Animal Care program, "Our investigators haven't expressed a concern. We have stopped screening at this point and have no plans to start again unless we have issues."
Other facilities have already made changes to their processes. The National Cancer Institute (NCI) Patient-Derived Models Repository (PDMR) decided to exclude MKPV from its animal facilities and tumor materials after an incident in which NSG mice became ill after infection with the virus. The PDMR receives human tumor materials from various academic labs, which are then implanted (in a special quarantine facility) into nude or NSG mice and expanded prior to cryopreservation of tumor samples for further distribution to the research community. The PDMR has a rigorous pre-screening process prior to sample receipt, but this particular sample was received prior to the publication on MKPV.
According to Melinda Hollingshead, DVM, PhD, Chief of the Biological Testing Branch which prepares cryopreserved tumors for the PDMR, "NSG mice into which tumor fragments were implanted became obviously sick. Pathology results showed the sick mice had inclusion bodies in the kidney. We got these results just after the paper describing MKPV came out, so we sent samples for diagnostics to our in-house lab and to IDEXX. The results showed the sick mice were positive for MKPV by PCR." For Dr. Hollingshead, the decision was simple. "We strictly exclude MKPV because it is an infectious agent that will cause harm to the strains of immunocompromised mice we utilize, and it could potentially be transmitted in the tumor material we distribute. Sending one bad sample could have a devastating effect on the recipient. We don't want to introduce an infectious agent into someone's facility."
Sharing of biological materials is a risk factor with regards to MKPV. Although the virus would not infect human cells, tumor materials can have sufficient residual mouse material to harbor the virus. The PDMR was able to identify that the original tumor fragment sent to them was positive for MKPV. The infected mice were humanely euthanized, and the facility was thoroughly decontaminated. Follow on environmental screening and sentinel testing showed that the PDMR was able to eliminate the agent from their facility. "The good news is that the virus did not blow through the facility. We were able to prevent spread through good technique and subsequently eliminate the agent from that facility. We have added MKPV to our screening protocol for all incoming material, and we test biweekly for it in our facility," said Dr. Hollingshead. "I think it's critically important to keep this agent out of a facility that has immunocompromised mice. Even for immunocompetent mice, experiments involving drugs may be compromised due to altered renal physiology in infected mice. Our goal is to facilitate improvements in human health. That is best done by building on research done in healthy research animals. These are the kinds of things that contribute to the lack of reproducibility of data, which is an ongoing struggle in the scientific community."
















.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)


.jpg)
.jpg)




.jpg)





.jpg)

.jpg)




