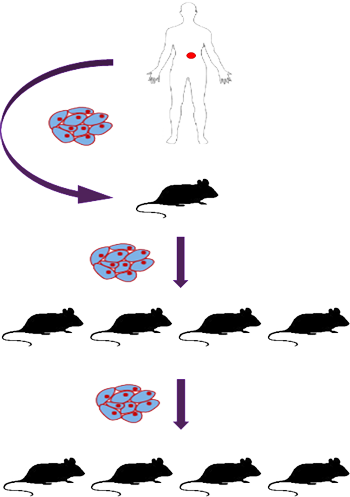
Dr. Els Hermans, whose work on the Trace platform at KU Leuven is streamlining the oncology R&D pipeline, presented a webinar focused on the application of patient-derived xenograft models (PDX) in translational cancer research.
Her presentation on use of PDX models to close the gap between pre-clinical and clinical trials, and their application in personalized medicine, generated substantial interest from the research community.
 View the Taconic Biosciences' Webinar:
View the Taconic Biosciences' Webinar:
There were more questions than could be answered during the live session, so we present additional Q&A here.
How long does it take for tissue collection from the patient to establish a patient-derived xenograft model?
This depends on tumor type. Validated melanoma models can be generated in 9-12 months as they grow quickly. For slowly growing tumor types, this can take longer. For example, the first xenograft implantation of breast, ovarian or endometrial tumors can sometimes take 8-9 months to grow to sufficient tumor volume. Subsequent passages typically grow more quickly, but the overall process can take 18 months to get the model established, and then it must be validated.
How much tissue is needed for a successful F1 take?
With more clinically aggressive tumors -- melanoma, pancreatic, head and neck, for example -- only a small amount of tissue is required. Pancreatic tumor PDX can be established from fine needle biopsies using 23 gauge needles, which yields a very small amount of tissue and can be implanted in a single mouse. More difficult types such as ovarian or endometrial cancers require larger starting tumor tissue, for example an implant of 2-3 mm
3 in each of four mice.
The first generation implant in mice is the tricky one; if the tumor successfully grows in the first implantation, it typically grows in successive passages.
What percentage of established models were from neoplasms and metastasis?
It depends on the tumor type and research project. Pancreatic xenografts are mainly derived from primary samples, while melanoma PDXs are established of lymph node metastasis (50-60%). It is very variable among the different groups.
Are tumor implants all performed subcutaneously, or does it depend on the tumor type? For example, are tumors implanted in the mammary gland?
At the moment, we transplant all samples subcutaneously since there is no request for orthotopic transplantation. Both approaches have their pros and cons: follow up, implantation technique, microenvironment, etc.
What is the best method to identify changes in the xenograft as compared to patient tumor?
Compare each PDX tumor to the original patient sample for histology (for the general morphology), combined with immunohistochemistry and fingerprinting based on a number of SNPs to verify the human background. This can be completed by next generation sequencing depending on the budget, the project and so on.
What are the limitations or drawbacks of culturing the tumor explants before implantation in mice?
In vitro culture of patient-derived cancer cells has been shown to deeply and irreversibly alter their gene expression profile and therefore their functional state (See
Daniel VC et al, Cancer Res 2009).
 View the Taconic Webinar:
View the Taconic Webinar:
 View the Taconic White Paper:
View the Taconic White Paper:






.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)
.jpg)


.jpg)


.jpg)




.jpg)

.jpg)
.jpg)






