Application Areas:
TK-NOG

| Model No. | Nomenclature | Genotype |
|---|---|---|
| 12907-F | NOD.Cg-Prkdcscid Il2rgtm1Sug Tg(Alb-UL23)7-2/ShiJic | sp/sp;ko/ko;tg/wt |
| 12907-M | NOD.Cg-Prkdcscid Il2rgtm1Sug Tg(Alb-UL23)7-2/ShiJic | sp/sp;ko/y;tg/wt |
- Description
- Price & Licensing
- Health Report
- Overview
- Genetics
- Guides & Publications
- Applications & Therapeutic Areas
- Transit, Housing & Welfare
- Diet
Overview
Nomenclature: NOD.Cg-Prkdcscid Il2rgtm1Sug Tg(Alb-UL23)7-2/ShiJic
- Also known as Alb-HSVtk-NOG and albumin-TK-NOG, super immunodeficient NOG mouse with transgenic expression of thymidine kinase under control of liver-restricted albumin promoter.
- Useful for humanizing liver via engraftment with human hepatocytes and/or humanizing immune system via engraftment of human immune cells/tissues or hematopoietic stem cells.
- Inducible ablation of murine hepatocytes by ganciclovir treatment enables stable, long-term engraftment of human hepatocytes.
- The premiere model for human liver toxicity, metabolism, and infectious disease.
- Other uses include safety and immunogenicity assessment.
- Please note the scientific nomenclature of this strain was updated in Sept 2021 to reflect most current allele designations.
“Experimental Pharmacology & Oncology (EPO) GmbH uses 1st and 2nd generation NOG mice provided by Taconic for its preclinical oncology service. These mice are included in novel concepts for the development of personalized treatment options and especially suited for humanization strategies. We are very satisfied with the quality of mice, the reliability of shipment and the high level of scientific support. Further, we very much appreciate the competent and always friendly communication.”
Recommended Controls
The recommended control for this model is CIEA NOG mouse.
Origin
Genetics
Guides & Publications
Initial Publication:
- Hasegawa M, Kawai K, Mitsui T, Taniguchi K, Monnai M, Wakui M, Ito M, Suematsu M, Peltz G, Nakamura M, Suemizu H. The reconstituted 'humanized liver' in TK-NOG mice is mature and functional. (2011) Biochem Biophys Res Commun. 18;405(3):405-10.
- Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, Tsuji K, Heike T, Nakahata T. (2002) NOD/SCID/γγ
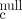 mouse: an excellent recipient mouse model for engraftment of human cells. Blood 100(9):3175-3182.
mouse: an excellent recipient mouse model for engraftment of human cells. Blood 100(9):3175-3182.
Applications & Therapeutic Areas
- ADMET
- Cell and Tissue Humanized
- Metabolic Disease
- Infectious Disease
Transit, Housing & Welfare
Need more info? Click the live chat button or Contact Us
Packing Practices
Taconic standard practice is to recombine animals of different home cages and/or ages from a single model and sex during packing, except in specific cases where Taconic's animal welfare policy prohibits recombination due to aggression or other concerns. When an order is fulfilled with animals from more than one week of birth, this standard practice results in animals from a range of birth weeks packed together in a single TTC. When an order is fulfilled with animals from genotyped models, this standard practice results in animals from different home cages packed together in a single TTC.
Customers who wish to keep animals from different weeks of birth separated should place orders with the special instruction "Divide and label by age." Note that this special request can result in increased costs for additional Taconic Transit Cages, dividers and/or freight charges.
Taconic discourages other types of custom packing requests as they can have a negative impact on animal welfare. Learn more.
Diet
- Licensing
- Pricing - USD
- Pricing - EUR
- Pricing - DKK
- Pricing - USD Nonprofit
- Pricing - EUR Nonprofit
- Pricing - DKK Nonprofit
- Select my Health Standard
- Get Custom Pricing Guide
TK-NOG
Nonprofit users (excluding users at nonprofit foundations which are affiliated with a for-profit entity): For internal research purposes, the CIEA NOG mouse® Conditions of Use for nonprofit users apply. If you wish to perform sponsored research or fee-for-service contract research using the CIEA NOG mouse®, please inquire for access conditions.
For-profit users and users at foundations which are affiliated with for-profit entities: The CIEA NOG mouse® Conditions of Use for for-profit users apply.
The CIEA NOG mouse® is produced and distributed under license rights to the following patents and trademarks:
- Japanese Patent No. 3,753,321
- US Patent No. 7,145,055; 5,464,764; 5,487,992; 5,627,059; 5,631,153; 5,789,215; 6,204,061; 6,653,113; 6,689,610
EP Patent No. 1,338,198 - Japanese Trademark Reg. No. 4,823,423
- US Trademark Reg. No. 3,118,040
- EU Trademark Reg. No. 3,736,758
Pricing - USD
Opportunist Free (OF) Health Standard
12907 Female
12907-F Genotype sp/sp;ko/ko;tg/wt
Cohorts are reserved upon order placement and will take 4-8 weeks to fulfill. An estimated lead time will be provided to you within 2-3 business days.
| Age in Weeks | Quantity 1 - 999 |
|---|---|
| 3 to 10 | US$631.00 |
12907 Male
12907-M Genotype sp/sp;ko/y;tg/wt
Available now
| Age in Weeks | Quantity 1 - 999 |
|---|---|
| 3 to 10 | US$631.00 |
Pricing - EUR
Opportunist Free (OF) Health Standard
12907 Female
12907-F Genotype sp/sp;ko/ko;tg/wt
Cohorts are reserved upon order placement and will take 4-8 weeks to fulfill. An estimated lead time will be provided to you within 2-3 business days.
| Age in Weeks | Quantity 1 - 999 |
|---|---|
| 3 to 10 | 574,00 € |
12907 Male
12907-M Genotype sp/sp;ko/y;tg/wt
Available now
| Age in Weeks | Quantity 1 - 999 |
|---|---|
| 3 to 10 | 574,00 € |
Pricing - DKK
Opportunist Free (OF) Health Standard
12907 Female
12907-F Genotype sp/sp;ko/ko;tg/wt
Cohorts are reserved upon order placement and will take 4-8 weeks to fulfill. An estimated lead time will be provided to you within 2-3 business days.
| Age in Weeks | Quantity 1 - 999 |
|---|---|
| 3 to 10 | kr.4.269,00 |
12907 Male
12907-M Genotype sp/sp;ko/y;tg/wt
Available now
| Age in Weeks | Quantity 1 - 999 |
|---|---|
| 3 to 10 | kr.4.269,00 |
Pricing - USD Nonprofit
Opportunist Free (OF) Health Standard
12907 Female
12907-F Genotype sp/sp;ko/ko;tg/wt
Cohorts are reserved upon order placement and will take 4-8 weeks to fulfill. An estimated lead time will be provided to you within 2-3 business days.
| Age in Weeks | Quantity 1 - 999 |
|---|---|
| 3 to 10 | US$491.00 |
12907 Male
12907-M Genotype sp/sp;ko/y;tg/wt
Available now
| Age in Weeks | Quantity 1 - 999 |
|---|---|
| 3 to 10 | US$491.00 |
Pricing - EUR Nonprofit
Opportunist Free (OF) Health Standard
12907 Female
12907-F Genotype sp/sp;ko/ko;tg/wt
Cohorts are reserved upon order placement and will take 4-8 weeks to fulfill. An estimated lead time will be provided to you within 2-3 business days.
| Age in Weeks | Quantity 1 - 999 |
|---|---|
| 3 to 10 | 446,00 € |
12907 Male
12907-M Genotype sp/sp;ko/y;tg/wt
Available now
| Age in Weeks | Quantity 1 - 999 |
|---|---|
| 3 to 10 | 446,00 € |
Pricing - DKK Nonprofit
Opportunist Free (OF) Health Standard
12907 Female
12907-F Genotype sp/sp;ko/ko;tg/wt
Cohorts are reserved upon order placement and will take 4-8 weeks to fulfill. An estimated lead time will be provided to you within 2-3 business days.
| Age in Weeks | Quantity 1 - 999 |
|---|---|
| 3 to 10 | kr.3.319,00 |
12907 Male
12907-M Genotype sp/sp;ko/y;tg/wt
Available now
| Age in Weeks | Quantity 1 - 999 |
|---|---|
| 3 to 10 | kr.3.319,00 |
Select my Health Standard
Need help choosing the right Taconic Biosciences health standard for your research?
Use the Health Standard Selector to enter your exclusion list. The tool will tell you which health standards meet your requirements.
Get custom pricing guide
Schedule A Scientific Consultation
Connect directly with a member of our Scientific Solutions team who can help you select the most appropriate model and maximize your experimental success.
















.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)


.jpg)
.jpg)




.jpg)





.jpg)

.jpg)











