Application Areas:
NFκB-RE-luc
- Description
- Data
- Price & Licensing
- Overview
- Genetics
- Guides & Publications
- Applications & Therapeutic Areas
- Transit, Housing & Welfare
- Diet
Overview
Nomenclature: BALB/c-Tg(Rela-luc)31Xen
- Carries a transgene containing 6 NFκB-responsive elements (RE) from the CMVα (immediate early) promoter placed upstream of a basal SV40 promoter, and a modified firefly luciferase cDNA (Promega pGL3)
- Basal expression of the reporter is observed in the whole body
- The reporter is inducible by LPS and TNFα
- The model provides for the rapid study of transcriptional regulation of the NFκB gene
- Useful in studying sepsis, arthritis, inflammatory bowel disease, apoptosis, tumor development, NFκB gene regulation, and the treatment of inflammatory diseases and cancer
Origin
The NFκB-RE-luc mouse was developed by Caliper Life Sciences. The model was created by microinjecting a transgene containing 6 NFκB-responsive elements (RE) from the CMVα (immediate early) promoter placed upstream of a basal SV40 promoter, and a modified firefly luciferase cDNA (Promega pGL3). This transgene was microinjected into BALB/cJ zygotes. The resultant mice from founder line 31 were bred to BALB/cJ mice. Taconic received stock from Caliper in 2010, and the line was embryo transfer derived. The line was maintained by mating mice which are wild type to those which are hemizygous for the luciferase transgene.
This model is cryopreserved and available for recovery. Models can typically be recovered and delivered to customers within 12 weeks after order receipt. Purchase of this model includes perpetual use rights and a deliverable of four mutant animals at the Murine Pathogen Free™ health standard along with a genotyping protocol. For models which include a recombinase gene or multiple alleles, all alleles will be provided, but individual animals may not contain all mutant alleles.
Taconic’s Colony Management experts can design a plan to grow your colony faster.
Genetics
Guides & Publications
Initial Publication:
There is no specific publication describing the generation of these mice, but multiple publications exist demonstrating applications using this model and a similar model generated by Dr. Blomhoff. See reference list.
Other publications:
- Carlsen H, Moskaug JØ, Fromm SH, Blomhoff R. (2002) In vivo imaging of NF-kappa B activity. J Immunol. 168(3):1441-6.
- Døhlen G, Carlsen H, Blomhoff R, Thaulow E, Saugstad OD. (2005) Reoxygenation of hypoxic mice with 100% oxygen induces brain nuclear factor-kappa B. Pediatr Res. 58(5):941-5.
- Alexander G, Carlsen H, Blomhoff R. (2006) Corneal NF-kappaB activity is necessary for the retention of transparency in the cornea of UV-B-exposed transgenic reporter mice. Exp Eye Res. 82(4):700-9.
- Sadikot RT, Zeng H, Joo M, Everhart MB, Sherrill TP, Li B, Cheng DS, Yull FE, Christman JW, Blackwell TS. (2006) Targeted immunomodulation of the NF-kappaB pathway in airway epithelium impacts host defense against Pseudomonas aeruginosa. J Immunol. 176(8):4923-30.
- Roth DJ, Jansen ED, Powers AC, Wang TG. (2006) A novel method of monitoring response to islet transplantation: bioluminescent imaging of an NF-κB transgenic mouse model. Transplantation. 81(8):1185-90.
- Ho TY, Chen YS, Hsiang CY. (2007) Noninvasive nuclear factor-kappaB bioluminescence imaging for the assessment of host-biomaterial interaction in transgenic mice. Biomaterials. 28(30):4370-7.
- Izmailova ES, Paz N, Alencar H, Chun M, Schopf L, Hepperle M, Lane JH, Harriman G, Xu Y, Ocain T, Weissleder R, Mahmood U, Healy AM, Jaffee B. (2007) Use of molecular imaging to quantify response to IKK-2 inhibitor treatment in murine arthritis. Arthritis Rheum. 56(1):117-28.
- Partridge J, Carlsen H, Enesa K, Chaudhury H, Zakkar M, Luong L, Kinderlerer A, Johns M, Blomhoff R, Mason JC, Haskard DO, Evans PC. (2007) Laminar shear stress acts as a switch to regulate divergent functions of NF-kappaB in endothelial cells. FASEB J. 21(13):3553-61.
- Dohlen G, Odland HH, Carlsen H, Blomhoff R, Thaulow E, Saugstad OD. (2008) Antioxidant activity in the newborn brain: a luciferase mouse model. Neonatology. 93(2):125-31.
- Notebaert S, Carlsen H, Janssen D, Vandenabeele P, Blomhoff R, Meyer E. (2008) In vivo imaging of NF-kappaB activity during Escherichia coli-induced mammary gland infection. Cell Microbiol. 10(6):1249-58.
- Vykhovanets EV, Shukla S, MacLennan GT, Resnick MI, Carlsen H, Blomhoff R, Gupta S. (2008) Molecular imaging of NF-kappaB in prostate tissue after systemic administration of IL-1 beta. Prostate. 68(1):34-41.
- Tukhvatulin AI, Logunov DY, Gitlin II, Shmarov MM, Kudan PV, Adzhieva CA, Moroz AF, Kostyukova NN, Burdelya LG, Naroditsky BS, Gintsburg AL, Gudkov AV. (2011) A In Vitro and In Vivo Study of the Ability of NOD1 Ligands to Activate the Transcriptional Factor NF-κB. Acta Naturae. 3(1):77-84.
- Morlacchi S, Dal Secco V, Soldani C, Glaichenhaus N, Viola A, Sarukhan A. (2011) Regulatory T cells target chemokine secretion by dendritic cells independently of their capacity to regulate T cell proliferation. J Immunol. 186(12):6807-14.
- Jung K, Lee JE, Kim HZ, Kim HM, Park BS, Hwang SI, Lee JO, Kim SC, Koh GY. (2009) Toll-like receptor 4 decoy, TOY, attenuates gram-negative bacterial sepsis. PLoS One. 4(10):e7403.
Applications & Therapeutic Areas
- Immunology
- Infectious Disease
- Inflammation
- Inflammatory Bowel Disease
- Microbiome
- Oncology & Immuno-Oncology
- Reporter Lines
Transit, Housing & Welfare
Need more info? Click the live chat button or Contact Us
Diet
Data
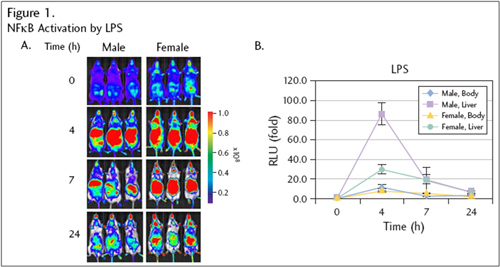
Figure 1. The mice (n=3) were imaged at T= 0 (pretreatment) and 4, 7, and 24 hours following intraperitoneal injection of LPS (1mg/kg) (A). Photons/sec quantified from liver and whole body. The data (B) represent mean fold of induction
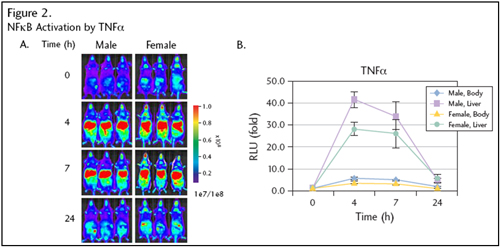
Figure 2. The mice (n=3) were imaged at T=0 (pretreatment) and 4, 7, and 24 hours following intraperitoneal injection of TNFα (2 ìg/mouse) (A). Photons/sec quantified from liver and whole body. The data (B) represent mean fold of induction.
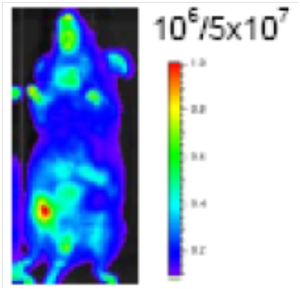
Figure 3. Basal expression. Ubiquitious expression in the whole body, strong signals in the abdominal and thoracic regions. The whole body signal intensity is 3x109 photons/sec.
Imaging Recommendations
Anesthetize mice prior to injection of luciferin and measurement of luciferase transgene expression. Optimal imaging occurs between 10 and 20 minutes following intraperitoneal injection of luciferin.- Licensing
- Pricing - USD
- Pricing - EUR
- Pricing - DKK
- Select my Health Standard
- Get Custom Pricing Guide
Licensing
This model is sold under terms which grant perpetual use rights.
Pricing - USD
10499-EZcohort-4
| Item | Commercial | Nonprofit |
|---|---|---|
| Cryopreserved Model | US$11,500.00 | US$11,500.00 |
Cryopreserved models are invoiced upon shipment of recovered animals. Once orders are placed, the full purchase price will be applied if the order is canceled. For orders greater than 4 animals, please contact Taconic for options.
Fees for Taconic Transit Cages™ and freight are in addition to the price above.
Pricing - EUR
10499-EZcohort-4
| Item | Commercial | Nonprofit |
|---|---|---|
| Cryopreserved Model | 9.950,00 € | 9.950,00 € |
Cryopreserved models are invoiced upon shipment of recovered animals. Once orders are placed, the full purchase price will be applied if the order is canceled. For orders greater than 4 animals, please contact Taconic for options.
Fees for Taconic Transit Cages™ and freight are in addition to the price above.
Pricing - DKK
10499-EZcohort-4
| Item | Commercial | Nonprofit |
|---|---|---|
| Cryopreserved Model | kr.74.227,00 | kr.74.227,00 |
Cryopreserved models are invoiced upon shipment of recovered animals. Once orders are placed, the full purchase price will be applied if the order is canceled. For orders greater than 4 animals, please contact Taconic for options.
Fees for Taconic Transit Cages™ and freight are in addition to the price above.
Select my Health Standard
Need help choosing the right Taconic Biosciences health standard for your research?
Use the Health Standard Selector to enter your exclusion list. The tool will tell you which health standards meet your requirements.
Get custom pricing guide
Schedule A Scientific Consultation
Connect directly with a member of our Scientific Solutions team who can help you select the most appropriate model and maximize your experimental success.
















.jpg)

.jpg)
.jpg)
.jpg)
.jpg)





.jpg)


.jpg)
.jpg)




.jpg)





.jpg)

.jpg)